by Gerald W. Hart, Ph.D., Teh-Ying Chou, Ph.D., Man-Shiow Jiang, Ph.D., Kenneth D. Greis, Ph.D., Robert N. Cole, Ph.D., Frank I. Comer, Chris S. Arnold, Tatsuji Matsuoka, Ph.D., Doris M. Snow, Bradley K. Hayes, Ph.D., Lisa K. Kreppel, and Betty J. Earles, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham. Hart presented this at the Glycobiology Interest Group's annual "Glycoday" on May 30, 1995, in Annapolis, Md.
ABSTRACT
About 11 years ago, we discovered that a very large number of nuclear and cytoplasmic proteins are modified by single N-acetylglucosamine (O-GlcNAc) residues that are O-glycosidically linked to serine and threonine moieties. Studies in several systems have shown that O-GlcNAc residues are both as abundant and as highly dynamic as phosphate groups. O-GlcNAcylation is also often reciprocal to phosphorylation and occurs at protein sites identical to those used by kinases that regulate cell growth (see figure).O-GlcNAc appears to be present in all eukaryotes, and has been postulated to play a key regulatory role in numerous cellular processes. Since these initial discoveries, our laboratory has been taking an eclectic approach to elucidate the functions of this ubiquitous form of intracellular protein glycosylation.
One part of this work examines transcription, in which O-GlcNAc appears to play a key role. RNA polymerase II (Pol II) and its transcriptional regulatory proteins are extensively modified by O-GlcNAc. Our data, together with those in the literature, suggest that the glycosylated, but not the phosphorylated, form of Pol II assembles in the initiation complex. Studies from several other labs further suggest that elongation of transcripts cannot take place until the C-terminal domain (CTD) of Pol II is extensively phosphorylated, which, according to our findings, would require its prior deglycosylation. Three recent results from our lab support a model of O-GlcNAc as a key player in transcription: 1) using synthetic O-GlcNAc-bearing CTD-derived glycopeptides as the substrate, we showed that O-GlcNAcase is present in transcriptionally active nuclear extracts, and its activity increases markedly with the addition of nucleotide triphosphates which also activate transcription; 2) using a highly specific adenovirus-2-major-late-promoter to drive transcription, we demonstrated that a highly specific inhibitor of N-acetylglucosaminidases also blocks Pol II-dependent transcription; and 3) we demonstrated that synthetic O-GlcNAc-bearing CTD glycopeptides but not the unmodified CTD peptides block transcription.

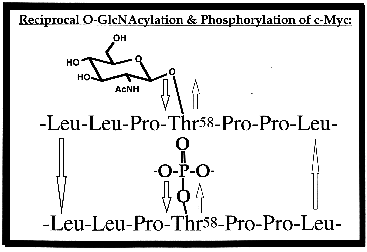
Another group of our studies examines nuclear proteins relevant to tumor growth. Using several methods, we showed that the oncoprotein c-myc, a helix-loop-helix, leucine-zipper phosphoprotein that heterodimerizes with Max and participates in the regulation of transcription in normal and neoplastic cells, bears O-GlcNAc residues in its N-terminal domain, a region involved in both transcription activation and malignant transformation. The major site of O-GlcNAcylation is Thr-58 (see figure), which is also the major phosphorylation site used by glycogen-synthetase-kinase-3 and is the major mutational "hotspot" in human lymphomas. Estrogen receptors, which are ligand-inducible transcription factors, are also modified by O-GlcNAc. An important site of glycosylation is in the PEST region (a sequence targeting the protein for degradation) of the carboxy-terminal F domain of the receptor. Data from our lab suggest that the nonglycosylated form of the receptor preferentially binds DNA. The human cytomegalovirus-tegument basic phosphoprotein, which appears to play a role in viral assembly, is glycosylated at Ser-921 and Ser-952. Importantly, we find that this protein, whether made by native virus or overexpressed in baculoviral-infected insect cells, is glycosylated at the same sites, validating the use of such overexpressed proteins in initial studies to localize O-GlcNAc sites on rare regulatory proteins. Recently, we have also shown that bovine brain casein kinase II alpha subunits contain O-GlcNAc. Studies are under way to evaluate the effects of glycosylation on kinase activity and subcellular trafficking of this regulatory protein.
The section of our work that focuses on cytoskeletal proteins centers on a protein called tau, which regulates microtubule assembly in normal brain cells and which we found to be extensively modified by O-GlcNAc. In the brains of patients with Alzheimer's disease, this protein becomes abnormally phosphorylated and as a result, polymerizes with itself to form the paired-helical filaments (PHF-tau) that make up the intracellular tangles that, along with extracellular plaques, typify the disease. Self-polymerized PHF-tau does not bind microtubules and does not function properly in their assembly. A major site of O-GlcNAc addition in normal tau is also a major abnormal phosphorylation site, accounting for 70% of the PHF-tau formation. These findings suggest the possibility that the defect in tau in Alzheimer's patients may not result from a defect in phosphorylation mechanisms, as is currently thought, but rather might result from a defect in O-GlcNAc regulation, with abnormal phosphorylation being a default process.
In other neuronal studies, we find that many neuron-specific proteins important to neurotransmitter release are both phosphorylated and contain O-GlcNAc. Using synaptosomal preparations, we are studying the role of O-GlcNAc in synaptic transmission and find that purified synaptosomes contain both the O-GlcNAc transferase and O-GlcNAcase. Synapsin I is a protein concentrated at nerve terminals that modulates neurotransmitter release by mediating the association of synaptic vesicles with the cytoskeleton in a phosphorylation-dependent manner, and we have mapped the O-GlcNAc residues on synapsin I to regions important for binding to synaptic vesicles or the cytoskeleton.
In another segment of our lab's work, we have constructed genes encoding a cytoplasmic form or a nuclear-targeted form of galactosyltransferase (GT, an enzyme that "caps" GlcNAc residues with galactose). When these genes are transiently transfected into Chinese hamster ovary (CHO) cells, the truncated GTs that they encode can only be detected within the first 12 hours, most likely because the cells die after that. In contrast, CHO cells transfected with the normal gene--encoding the full-length enzyme active in the Golgi lumen--survive for long periods. Available data suggest that cytoplasmic or nucleoplasmic expression of GT is a lethal event, perhaps due to O-GlcNAc-capping or to the binding of GT to O-GlcNAc proteins.
Q: What was your starting point in this research, and how have your questions evolved?
A: In 1983, Carmen-Rosa Torres, a graduate student in our laboratory, was using highly purified glycosyltransferases to probe the complex glycosylation of proteins in murine immune system cells. When she probed lymphocytes with bovine milk galactosyltransferase and UDP-[3H]galactose to measure GlcNAc-terminating glycans, she found, surprisingly, that nearly all of the label attached to N-acetylglucosamine monosaccharides that are O-glycosidically attached to Ser(Thr) residues--a linkage not previously known to exist. We have gradually progressed away from complex glycans on cell-surface receptors, and currently, virtually our entire laboratory is studying the function of O-GlcNAcylation.
Q: Which findings have been most surprising to you or to other scientists?
A: Virtually all O-GlcNAcylation occurs on nuclear and cytoplasmic proteins. Before O-GlcNAc was discovered, dogma held that cytoplasmic and nuclear proteins were not glycosylated. The most surprising finding is the very large number of important nuclear and cytoskeletal proteins that are O-GlcNAcylated.
Q: What were the greatest stumbling blocks, and what new observations, techniques, reagents, or insights helped you get past them?
A: The greatest stumbling blocks were the development of sensitive techniques for the detection and quantification of O-GlcNAc on low-abundance regulatory proteins. A further complication is that virtually all eukaryotic cells contain an abundance of hexosaminidases that rapidly remove O-GlcNAc whenever cells are damaged. The development of potent O-GlcNAcase inhibitors, improved site-mapping techniques by HPLC, gas-phase sequencing, mass spectrometry, and capillary electrophoresis have significantly improved our ability to study O-GlcNAcylation of regulatory proteins.
Q: How can clinical scientists capitalize on this research?
A: It is our belief that O-GlcNAcylation may turn out to be as fundamental and as important to cellular regulation as protein phosphorylation. The O-GlcNAcylation of oncogenes and tumor suppressors opens up unexpected avenues for cancer therapy. Inhibitors of O-GlcNAcases could potentially be valuable in the treatment of Alzheimer's disease. The hyper O-GlcNAcylation of transcription factors could play a role in abnormal regulation of insulin expression in certain types of diabetes. As we continue to gather more fundamental data, the reality and application of these speculations will become evident.
Q: How are you following up on this work, and what questions would you ultimately like to answer?
A: Everyone in our laboratory is focused on determining the functions of O-GlcNAcylation. Some questions we are most concerned with are, Is O-GlcNAc a regulatory modification, analogous to phosphorylation? Does it have a reciprocal function with respect to phosphorylation on most proteins? How is O-GlcNAcylation or de-O-GlcNAcylation regulated? What specific role(s) do the O-GlcNAcylation of RNA polymerase II and its transcription factors play in the regulation of cell-type-specific gene transcription?