
| T H E N I H C A T A L Y S T | J U L Y – A U G U S T 2005 |
|
|
|
Translational
Research Success Story
|
by Celia Hooper |
 |
|
Warren
Strober (left) and Peter Mannon
|
Increasingly successful treatment of a devastating pair of chronic diseases, Crohn’s disease and ulcerative colitis (UC), stands as an elegant demonstration of the bench-to-bedside cooperation that NIH boasts.
Presenting the work of scientists in NIAID’s Mucosal Immunity Section of the Laboratory of Host Defenses, Peter Mannon and his section chief, Warren Strober, recently described gratifying clinical progress in treating two forms of inflammatory bowel disease (IBD). The pair spoke at NIH’s "Demystifying Medicine" course in early May.*
Bedside breakthroughs sprang from the emerging concept of IBD as a Kafkaesque variant on autoimmune disease in which a hyperactive immune system relentlessly and inappropriately reacts not to oneself, but to the next closest thing—the bacteria that make up part of the gut flora. Crossfire from the ensuing battle—and attempts to intervene—wreaks havoc with a patient’s gut and other tissues.
By modulating key regulatory cytokines to tone down the reactivity of the immune system, Strober’s lab has been able to eliminate IBD in mouse models of the disease—paving the way for translation to humans. Early-stage trials offer some promise of remissions in Crohn’s and UC patients.
The Clinical Picture of Crohn’s
Crohn’s disease, occurring in 40 to 100 of every 100,000 people, is a chronic disease unfolding from inflammation of the gastrointestinal (GI) tract, primarily patches of the small bowel and colon. As Mannon describes it, inflammation of the GI tract can lead to reactions in other tissues, including eye, skin, and bone. Ongoing symptoms may include formation of fistulae and fibrotic obstructions, abdominal pain, nutrient malabsorption, fever, bleeding, oral ulcers, kidney stones, arthritis, uveitis, and a variety of skin lesions.
Data from 1990 put the direct cost of Crohn’s at more than $1 billion, with the high toll, Mannon says, reflecting Crohn’s tendency to strike young adults and to recur. The disease and the side effects of standard treatments—corticosteroids, 5-aminosalicylic acid (5-ASA), and immunomodulators such as azathioprine and methotrexate—often make work impossible and life miserable. Up to 74 percent of patients eventually need surgery to clear gut obstructions and other complications. Repeated removal of segments of the small bowel in turn may lead to additional nutrient absorption problems.
| FIGURE 1 |
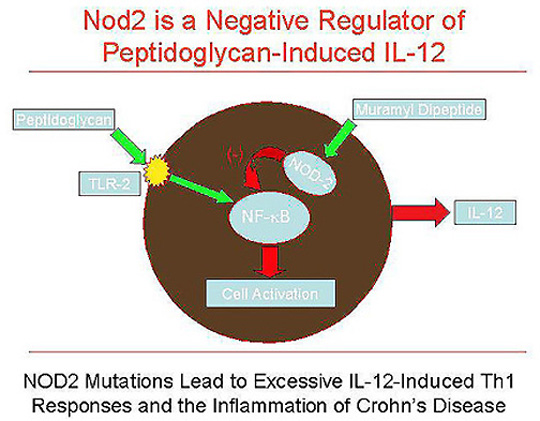 |
The Clinical Picture of UC
Similarly disabling, chronic, and relapsing, UC is an inflammation of the lower reaches of the bowel, from the distal rectum up into parts of the colon. Continuous rather than patchy in its distribution, UC affects only the lining of bowel, without forming fistulae knotting into other tissues. Mannon says the onset of UC tends to be more abrupt than Crohn’s, but many of the general symptoms are similar, including diarrhea, pain, and weight loss. Proctitic symptoms, such as rectal pain, urgency, and bloody stools, are more prominent in UC; affected tissues outside the GI system include the eye, skin, joints, and liver. Patients are at risk for colon cancer and inflammation of the biliary system. Two-thirds will develop chronic relapsing disease, and 40 percent will undergo surgery for UC. Current treatments include topical and oral 5-ASA, corticosteroids, and immunomodulators.
From Mouse Models of IBD . . .
A view of IBD as an immune disorder began to emerge 30 years ago. In the past decade, Strober’s lab developed animal models that greatly refined that view, framing Crohn’s as a Th1-mediated immune disease heavily influenced by IL-12, and UC as a Th2-like disease (like but not identical to a Th2-mediated response) heavily affected by IL-13. In both, inflammatory cells in the gut churn out their respective cytokines in response to normal gut flora.
Gut bacteria were first implicated as immune system provocateurs by the fact that germ-free animals could not be induced to develop IBD, no matter what immune system genetic lesion researchers visited upon them. But IBD can be induced via gene manipulations in animals that harbor any of a large number of different gut bacteria or are exposed to muramyl dipeptide, a component of some bacterial coats. Strober says that most of the thousands of antigens of the gut flora are benign—but the species that can potentially trigger IBD "may number in the hundreds and may vary from individual to individual," so that identifying the exact culprits for a particular IBD patient is nearly impossible.
Strober’s lab used knockout mice to piece together the hypotheses that Crohn’s disease (see Fig. 1) involves various cytokines and NF-kB, NOD-2, and TLR-2, with dysregulation of IL-12, IFN-g, and TNF-a.
Strober’s murine model of UC uses oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one)—a contact-sensitizing agent applied to the rectum. Much like an adjuvant in a vaccine, the chemical spurs the immune system to respond to the flora that line the lower reaches of the bowel. The treated mice exhibit UC-like symptoms, microscopic pathology, and changes in cytokines.
Strober’s group was able to block colitis symptoms by neutralizing IL-13 or thwarting presentation of antigen through interference with CD1 on natural killer T (NKT) cells. They now see UC as a Th2-like inflammation with IL-13 playing the lead role (see Fig. 2).
| FIGURE 2 |
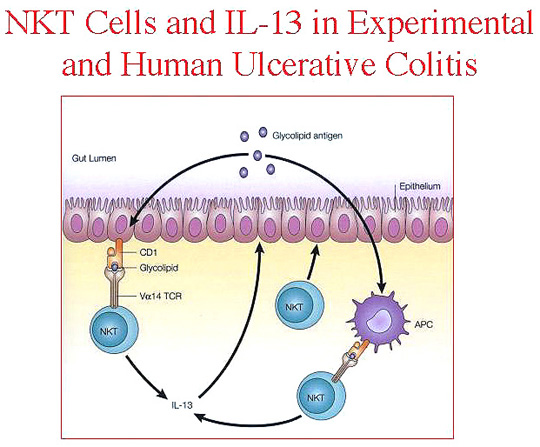 |
. . . To Clinical Trials of Cytokines
Crohn's: Strober says studies of cells from Crohn’s patients confirmed that immune cells in the gut mucosa secrete increased amounts of IL-12 and other effector cytokines, including IFN-g and TNF-a. He believes IL-12, the master cytokine of Th1 T-cell differentiation, drives the hyperactive immune response in Crohn’s.
Based on these and other studies, physicians in the past few years have been using infliximab, an anti–TNF-a monoclonal antibody, to treat Crohn’s, enlarging the percentage of patients with remissions and launching a new era of increasingly focused immune therapies. Other agents under investigation promise to increase the frequency, durability, and extent of response, while reducing side effects, Mannon says. These include IL-10; ISIS-2302 (an antisense oligonucleotide to ICAM-1); an anti–IL-6 receptor monoclonal antibody; thalidomide; granulocyte and granulocyte-macrophage colony-stimulating factor (G-CSF/GM-CSF); and anti–IFN-g.
An NIH study group that includes Strober, Mannon, and Ivan Fuss completed a pilot phase 2 trial that tested an antibody against IL-12 in adult patients with active Crohn’s disease. The multicenter, randomized, controlled, double-blind tr
ial of 80 patients included 63 who received human monoclonal antibody directed against the p40 subunit of IL-12. The group tested two dose levels and two admin-istration schedules for seven injections of antibody. The treatment was well tolerated, and one of the schedules for the higher dosage led to significant responses and remission of disease compared with placebo (NEJM 351:2069–2079, 2004).
Mannon was impressed that the patient response was as good as or better than that with infliximab. He suspects it would be possible to obtain even better results with refinements in dosing and maintenance regimens. "It’s a very promising approach," Mannon says. The pharmaceutical company that owns rights to the drug is examining application to other diseases. NIH investigators have started a pilot phase 2 study of G-CSF as another approach to down-modulating IL-12 and perhaps inducing T-regulatory cells in Crohn’s patients.
Ulcerative Colitis: Investigational approaches to treating UC include:
![]() Dietary supplements and probiotics ingested to alter the gut flora
Dietary supplements and probiotics ingested to alter the gut flora
![]() Granulocyte pheresis
Granulocyte pheresis
![]() Topical epithelial growth factor
Topical epithelial growth factor
![]() Anti–IL-13 agents
Anti–IL-13 agents
![]() Anti-CD3 and anti–IL-1
Anti-CD3 and anti–IL-1
Strober says that one of the most promising investigational drugs for UC, IFN-b, may work in accordance with his lab’s model—by blocking NKT cell activity, thereby curtailing IL-13 production. Mannon says the group has now recruited more than half of the patients needed for its pilot phase 2 study of IFN-b. A UC patient in the open-label trial describes prolonged periods without disease relapse, and Mannon says the early results are "very encouraging."
Strober would like to see the development of a UC therapy based on more direct, specific inhibition of IL-13. Mannon sees the future of UC treatment in detailed, individualized understanding of each patient’s dysfunctional immune relationship with his or her gut flora. Treatment would be targeted to identified patient subsets; a lab test would predict relapse risk, informing decisions for early intervention.
Beyond IBD
Strober and Mannon believe concepts emerging from their work may have application beyond IBD. Intervention against IL-12—also at work in rheumatoid arthritis, multiple sclerosis, and psoriasis—might be effective in suppressing these other Th1 immune-mediated diseases.
Based on observations
of Th2-like aspects of UC, investigators are looking at the involvement of IL-13
in allergic reactions and asthma. But UC’s most important lessons may be
more conceptual, Mannon says, as it involves a distinctive interplay of cytokines
and cells at work. The tiny population of NKT cells that manage to cause such
a large immune malfunction offers "a real opportunity to target therapy
not broadly, but to a very specific population of immunocytes that seem to be
the bad actors," Mannon says, emphasizing the importance of focusing therapy
on more upstream factors in the cascade of cytokines in immune responses. ![]()
-----------------------------------------------------------------------
* Webcasts of the course are available at this site.
Course materials are on the web at this site.