| T H E N I H C A T A L Y S T | J U L Y – A U G U S T 2003 |
|
|
|
SARS
Coronavirus Could Resurface in Chilly Weather
|
by Fran Pollner |
 |
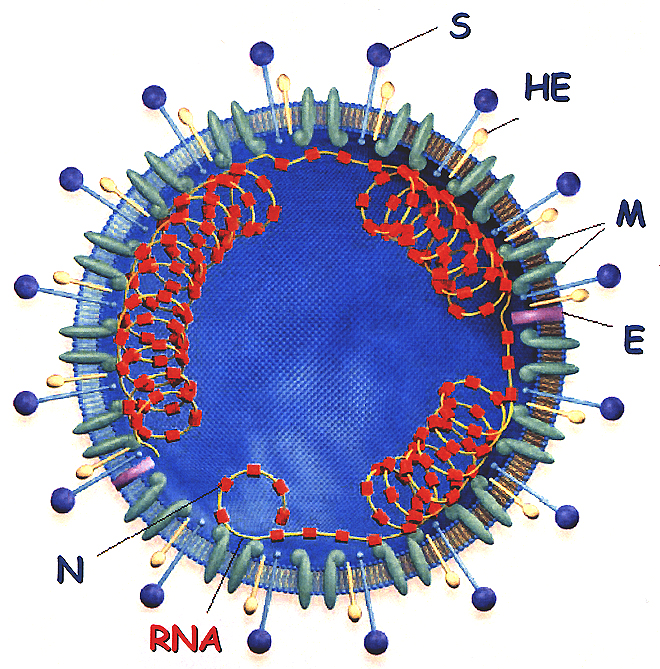
| Kathryn Holmes, "SARS-Associated Coronavirus, NEJM 348: 1948-1951, May 15, 2003; shown also during her talk on coronavirus virology and pathogenesis at the NIH workshop May 30 |
|
Structure
of the Coronavirus Virion: S = spike glycoprotein (the viral fusion protein),
HE = hemagglutinin-acetylesterase glycoprotein, M = membrane glycoprotein,
E = small envelope glycoprotein, N = nucleocapsid phosphoprotein
|
The chain of events that led to a World Health Organization warning against traveling to Toronto began with the hospital spread of SARS from a patient and his wife. The couple passed the virus to 84 people in 31 hours.
Most of those infected, said Allison McGeer, director of infection control at Mount Sinai Hospital and a professor at the University of Toronto, had been "within droplet range for no more than one to two minutes."
Within days of this event, which turned out to be a rare example of a SARS "superspreader," 19 Toronto hospitals were affected by SARS, and every one of them wanted to be able to get a clinical trial going, McGeer recounted during a clinical research breakout session at the international SARS colloquium held at NIH May 30.
"But there was no way they could get a clinical trial protocol through the ethics boards of 19 hospitals before the outbreak, which lasted a month, was over—not to mention that it’s hard to focus on anything but treating during an outbreak. This is a major challenge," she said.
Many agreed that new mechanisms are needed for rapid—but careful—approval of national and international protocols to respond to such precipitous events.
 |
| Teamwork: (left to right) Henry Masur, CC critical care chief and an associate investigator in the CC/NIAID SARS protocol; John Beigel, CC clinical research fellow and protocol PI; and Cliff Lane, NIAID clinical director and the protocol's accountable investigator |
Meeting the Challenge
Investigators generally view the next cold-weather flu season as a likely context for SARS resurgence. Collaborating scientists from NIAID and the CC are seeking IRB approval of a SARS clinical research protocol that would begin the instant the first patient in the metropolitan area thought to have SARS is referred to NIH as a study candidate.
The protocol encompasses three study cohorts comprising up to 200 individuals: SARS patients, their household contacts, and CC health-care workers.
"A major issue," said Henry Masur, chief of critical care medicine at the Clinical Center, "is how the virus is spread, and a major thrust of the study is determining from where, how much, and for how long the virus is excreted."
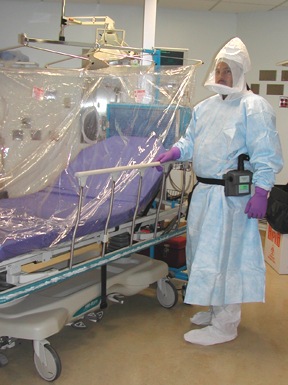
The protocol targets patients who have relatively mild disease when they are invited to enroll. It’s anticipated that most of the patients would drive themselves to the Bethesda campus and be met at the perimeter and transferred via a dedicated negative-flow transport device (see photo below). They wouldl enter the hospital through a special entrance and be taken directly to a negative-flow room.
Blood, urine, feces, and respiratory secretions would be analyzed on a regular basis. Among the study goals listed in the protocol are:
![]() To evaluate and treat persons with SARS
To evaluate and treat persons with SARS
 |
|
Private
Spaces:
The DeMistifier rolling bed/isolation unit, acquired to transport a SARS
protocol patient from his or her vehicle to a secure CC entrance and up
to the negative-flow room where the patient would be cared for—with
CC respiratory
therapy chief Dennis
Brown in attendance, as would often be the case throughout the upcoming
study. Brown is wearing the powered air-purifying respirator (PAPR) and
protective gown, gloves, and foot gear that all involved health-care workers
would don while participating in the study. Filtered air is delivered
to the hood by tube from a pack worn around the waist; the hood is disposable
and after each patient encounter would be discarded in the anteroom outside
the negative-flow room. Brown says he is not too worried about his personal
safety: "I wouldn’t have signed on as a health-care provider—especially
at NIH—if I didn’t know some risk was involved." Brown
and others, including hospital epidemiologist David
Henderson, believe this risk is minimal based on the ability of hospitals
in Asia and Toronto to control SARS once they understood and enforced
the necessary precautions.
|
![]() To elucidate the pathophysiology of SARS
To elucidate the pathophysiology of SARS
![]() To characterize the immune response during SARS
To characterize the immune response during SARS
![]() To evaluate diagnostic tests for the rapid identification of SARS in clinical
specimens
To evaluate diagnostic tests for the rapid identification of SARS in clinical
specimens
Contacts: Household and Hospital
The second study population of family members and other close contacts would be enrolled and followed serially for evidence of the onset of viral excretion and correlation with the presence—or absence—of clinical symptoms. Data generated from this cohort should expand knowledge of the natural history of the disease. "We’d have an unparalleled opportunity to study disease development virlogically and immunologically from very early after exposure."
|
'We announced in April that we might bring SARS patients to the Clinical Center to participate in a study. We felt we have a fabulous environment scientifically—and a safe environment—to study SARS. But that possibility generated more e-mail to me than anything else in the eight years I’ve been Clinical Center director.' 'The facts of the evolution [of the SARS outbreak] show that when the medical staff is prepared, the chain of transmissibility is broken. The risk of spread in a hospital that is conducting a study is low.' CC Grand Rounds, June 18, 2003 |
The third population—NIH health-care workers who come in contact with the study patients—would be followed to "make sure they are not acquiring the virus.
"While the risk of a hospital worker becoming infected is remote, that possibility does exist," Masur said. "Should that happen, the person would be moved into the other protocol group—but the hospital leadership and NIAID leadership have confidence in the caliber of our facilities and our staff and our ability to handle this virus better than can be done almost anywhere else." He noted that hospital epidemiologist and CC deputy director David Henderson, an associate investigator in the study, has discussed SARS transmission with involved hospital authorities in Canada and Asia and has concluded that the risk at the Clinical Center should be manageable, as has Cliff Lane, NIAID clinical director and the accountable investigator on the protocol.
The far greater danger to any hospital staff and to other patients, protocol
leaders have observed, would be exposure to CC patients and staff who develop SARS through travel or personal contact and then enter the building for work or for treatment on protocols not related to SARS.
"Perhaps the most important lesson learned from the Toronto experience," Henderson said, "is that health-care workers at the front-line in an epidemic must have extremely high indices of suspicion for the diagnosis and proper management of highly contagious diseases such as SARS. Almost all instances of transmission occurred in settings in which the disease was not suspected." Conversely, once the disease was recognized, even institutions in developing countries with limited resources were able to contain the infection by careful adherence to traditional infection-control procedures.
Aside from CC excellence in infection control, other factors that argue for clinical SARS research at the CC are the NIH tradition and responsibility to respond to national health priorities, protocol investigators agree. They point to the response of the CC to the AIDS epidemic in the early 1980s, when there was considerable fear about having patients with HIV in hospital settings, as an example of meeting that obligation and advancing science and patient care in a perilous time.
Beyond the First SARS Protocol
The CC/NIAID investigators also envision two additional studies that could culminate in the development of a novel SARS therapy. The first would entail identifying patients who have recovered from SARS and bringing them to the Clinical Center to obtain plasma samples from them.
"The idea is that we can use these samples to make an immune serum globulin. We know that SARS patients make antibody to the virus," Masur said. "We would harvest that antibody, concentrate it, and remove potentially infectious elements." This potential product would be tested in a variety of in vitro studies and, if results warranted and the FDA approved, would then move into human testing.
In the current protocol, treatment is described in flexible terms:
"As there is no clear optimal therapy, and the data regarding optimal therapy for SARS [are] continually changing, two options will be presented to the patients regarding therapy. First, the patients may be treated as clinically indicated by best medical practice given our continually changing knowledge of SARS.
"Alternatively, patients
may be enrolled in other protocols designed to evaluate therapies. These may
be intramural protocols of the NIH or protocols from outside study groups for
which the NIH is a collaborative center." ![]()
| SARS:
IMPACT AND RESPONSE SWIFT AND W0RLDWIDE |
|||||
A critical mass of international scientists converged at NIH May 30 to construct a research agenda to defeat severe acute respiratory syndrome (SARS). The global scourge had infected 8,439 people and killed 812 by the time the first recognized outbreak was declared contained a month later. A global report from the World Health Organization, first-hand accounts of the Hong Kong and Toronto outbreaks, and lectures on coronavirus biology set the stage for five concurrent working groups to hammer out research recommendations by the end of the day. This research agenda was speedily transferred to cyberspace at this site, which also displays all the slides presented throughout the day; the plenary talks can also be viewed here.
|
|||||