
| T H E N I H C A T A L Y S T | J A N U A R Y – F E B R U A R Y 2002 |
|
|
|
MIGHTY MACHINES FOR MINI-MODELS:ALL SYSTEMS GO FOR NEW MOUSE IMAGING FACILITY |
 |
|
Not
the Least Miffed (l to r): Felix
Onojafe, biologist; Daryl
Despres, biologist; Brenda
Klaunberg, research veterinarian;Alan
Koretsky, director;
Marty Lizak, MRI physicist; Alan
Olson, engineer; Lalith
Talagala, technical director
|
The mouse has always had a central place in studies of human health and disease; but in the age of the mouse genome and transgenic and knockout mouse models, its standing and ubiquity in basic and clinical research are beyond measure.
Just about everything else in the mouse, however, is measurable—and visible in all its aspects, with the right tools.
NIH has now amassed those tools and centralized them in a state-of-the-art home that reflects the status of the mouse in biomedical research.
The Mouse Imaging Facility (MIF), nestled at the end of a labyrinthine series of art-covered corridors in the B1 level of the Clinical Center, is now ready to accommodate NIH’s substantial mouse biology community. (It will also be open for general viewing March 5; see "Open House" below).
| BECKER'S BRAINCHILD |
| Nary
a soul involved in the MIF does not trace the facility’s origins back
to Ted Becker,
former NIDDK investigator and associate director for research services,
who was the driving force behind the idea and creation of what in 1985 was
called the In Vivo NMR Center. Says Becker: "Basically, I brought all
the SDs together and asked for money." That model holds to this day.
|
The MIF is an NIH-wide shared resource to which all intramural scientists have access. In 1998, it was an idea whose time had come, pushed to the fore by NHLBI’s Bob Balaban and then–CC Radiology’s Nick Bryan. In 1999, it was still an idea, but it had a director, Alan Koretsky, recruited by NINDS to help oversee its realization. In 2000 and 2001—the first two years of a three-year pilot project funded by participating NIH institutes in proportion to their intramural budgets—quarters were renovated, people were recruited, committees were formed, and equipment dedicated to the imaging of small animals was secured.
Now investigators who walk through the door of the MIF will find a cornucopia of modalities optimized for imaging small rodents at high resolution: ultrasound, computed tomography (CT), magnetic resonance imaging MRI), positron emission tomography (PET), and laser Doppler. A luciferase imager is on order.
MRI, however, is the modality "nearest and dearest" to his heart, says Koretsky, who also directs the MIF parent facility, the NIH MRI Research Facility (NMRF); he is also chief of the Laboratory of Functional and Molecular Imaging, NINDS, which has its own small animal MRI resources. Koretsky heads two ongoing protocols—one exploring cellular energy metabolism in the rat liver, heart, and brain and the other imaging brain function in rats and mice.
These two are among 30 animal studies in progress in the NMR Center, about one-third of which involve mice and rats. It was anticipated that some mouse studies begun in the center’s 4.7-tesla MRI would be moved to the MIF’s new 7-tesla machine.
In a progress report to the scientific directors late last year, Koretsky announced that the MIF would indeed be operational the first month of the new year, and he showcased some examples of MRI studies:
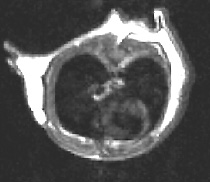
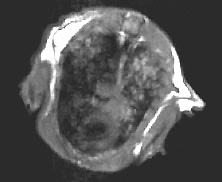
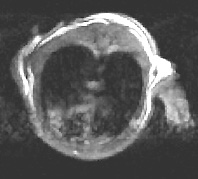
![]() Serial tracking of inducible lung cancer in a mutant mouse model (Galen
Fisher, NHGRI, and Marty
Lizak, MIF; see Figure 1)
Serial tracking of inducible lung cancer in a mutant mouse model (Galen
Fisher, NHGRI, and Marty
Lizak, MIF; see Figure 1)
![]() Dynamic MRI to assess tumor neovasculature (Steve
Libutti, NCI)
Dynamic MRI to assess tumor neovasculature (Steve
Libutti, NCI)
![]() Monitoring genes injected into the rat heart (Jonathan
Sorger and Elliot
McVeigh, NHLBI)
Monitoring genes injected into the rat heart (Jonathan
Sorger and Elliot
McVeigh, NHLBI)
![]() Tracking macrophage infiltration into the ischemic kidney (Robert
Star, NIDDK)
Tracking macrophage infiltration into the ischemic kidney (Robert
Star, NIDDK)
![]() Tracking the course of tagged neural stem cells (Joe
Frank, CC)
Tracking the course of tagged neural stem cells (Joe
Frank, CC)
The first of these—tracking lung tumors—Koretsky later observed, "is a good example of imaging that helps a sophisticated mouse molecular biology study, and it represents something that we can do routinely. The other MRI studies are in a more developmental stage.
Other MIF modalities being used by NIH investigators include high-resolution ultrasound, harnessed by NHLBI’s Cecilia Lo to elucidate cardiac dynamics and embryo surgery (see Figure 2), and micro-CT, which provides superb contrast between bone, soft tissue, and fat (see Figure 3). MIF research veterinarian Brenda Klaunberg and NIDDK’s Marc Reitman are developing protocols for routine regional fat determinations using CT. Also, Koretsky noted, the MIF is helping develop a microPET resource for NIH—a working prototype (ATLAS) built by the CC’s Michael Green and his colleagues (see Figure 4). PET is especially useful, Koretsky said, for detecting specific molecular interactions in vivo, such as neurotransmitter receptor distribution. Once the prototype is standardized, it will be moved into the MIF, he said.
   |
|
Some
Tools of the Trade
(l to r):
the
7-tesla, 21-cm mouse-imaging MRI, late last year before the machine was
actually installed; micro-CT, ultrasound
|
   |
|
The
MIF has surgical and physiological monitoring capabilities: (right) mouse
prep area; (center) Tonya
Hopkins, CC, monitors MRI tracings; (left) Mouse holding area: two
laminar flow rooms hold 95 cages; animals may remain for a few days only
|
Expert advice on which modality would best serve any given research objective is part of the MIF package of resources for investigators new to the field.
Oversight of the MIF comes under the NMR Center Steering Committee, chaired by Balaban. Balaban established the MIF subcommittee, which is chaired by King Li, the new head of the CC radiology department.
In addition to the animal imaging advisory subcommittee, there are MIF subcommittees to oversee animal safety and to review research proposals for their technical feasibility and the extent to which they will require MIF resources, including imaging time, ancillary equipment, and technician support. MIF structure and procedures can be found at its web site.
It is not the MIF’s job, however, to assess the scientific merit of proposed projects or to evaluate issues related to animal experimentation—those tasks reside within each institute and, more specifically, with its SD and its animal care and use committee. Before investigators present a protocol to the MIF, they must have done the legwork to secure those approvals.
Currently, each institute supports the MIF with a share proportional to its intramural budget. Once the pilot phase of the MIF is concluded, the funding formula that has sustained the NMR Center will go into effect in the MIF: 25 percent of the total budget will continue to be based on intramural budget and 75 percent will be based n facility usage. For the NMR Center, allocations for the coming year are estimated on the basis of usage from the previous three years, but there is a good deal of flexibility based on real-time needs as they arise. Tracking historical use of the MIF is just beginning.
Koretsky believes investigators involved in the numerous mouse studies across ampus will find MIF resources invaluable for analyzing a variety of phenotypes.
Li sees the MIF as a critical
locus in bench-to-bedside research in the postgenomic era. The "challenge
of medical imaging," he says, "is to be able to provide in vivo information
at the molecular level in a spatially and temporally resolved manner. To achieve
this, in vivo experiments in animal models are essential." ![]()
 |
 |
 |
|
wild-type
mouse with no lung tumor
|
lung
of mutant mouse model after three months on doxycycline
|
the
same model eight days after doxycycline withdrawal
|
|
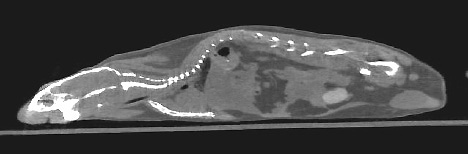
Figure 1. MRI of Inducible Lung Tumors: Mutant K-Ras transgene whose expression could be controlled with doxycycline caused tumors in an Ink/Arf knockout background. The tumors regressed when the expression was switched off. —Galen
Fisher, NHGRI, |
||
 |
|
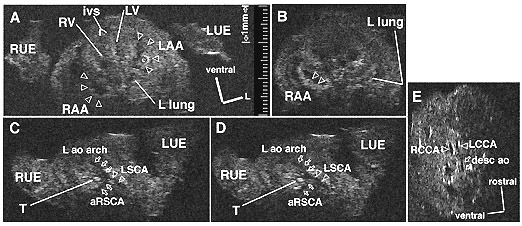
Figure 2. Ultrasound of a Mouse Embryo: Ultra-high-frequency (55 MHz) ultrasound imaging of an ex utero, fixed, tbx1 +/- mouse e13.5d embryo. A. Transverse image showing the heart within the thorax, flanked by the right (RUE) and left (LUE) forelimbs. The interventricular septum (ivs) points ventrally, resulting in the ventricular apex’s appearing mesocardic at this stage. The ventricles are ventral to the atria. An echo-dense thrombus (starburst) is seen within the left atrium. The hilum of the left (L) lung can be seen. (LAA = morphologically left atrial appendage; LV = left ventricle; RAA = morphologically right atrial appendage; RV = right ventricle; 1 mm calibration is shown.) B. Transverse image of the heart (same embryo) slightly inferior to that in A. Pectinate muscle ridges (open arrowheads) within the morphologically right atrial appendage can now be recognized. The distal aspect of the left lung can also be seen. C,D. Transverse images at planes more cephalad than in A. The left aortic (L ao) arch is visualized to the left of the trachea (T). An aberrant (retro-esophageal) right subclavian artery (aRSCA) and a normal left subclavian artery (LSCA) can be identified. E. Sagittal imaging through the left aortic arch reveals the right (RCCA) and left (LCCA) common carotid arteries and a portion of the descending (dorsal) aorta (desc ao). —Cecilia Lo, NHLBI, and Alvin Chin, Children’s Hospital of Philadelphia |
 |
 |
 |
|
|
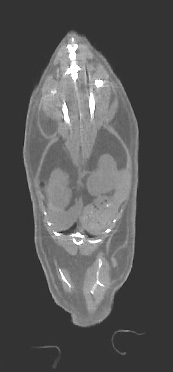
Figure 3. X-ray Computed Tomography (CT): These images of a mouse were acquired at ~100 micrometer resolution. CT images are excellent for characterizing bone (brightest intensity), organ size and distribution (intermediate intensity), and regional fat (lowest intensity). —Brenda Klaunberg and Alan Olson, MIF |
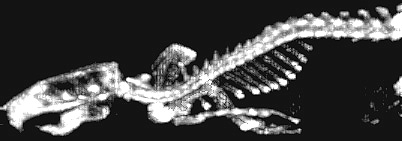 |
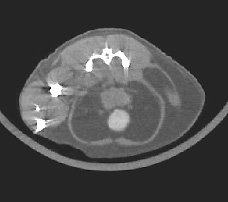
Figure 4. Micro-PET: Whole-body re-projection image of the rat skeleton two hours after intravenous administration of F-18 fluoride, a bone-seeking PET radiopharmaceutical. The full 3-D tomographic image set from which this image was synthesized was obtained with ATLAS, a small animal PET scanner designed and fabricated at NIH. —Mike Green, CC |