| T H E N I H C A T A L Y S T | S E P T E M B E R – O C T O B E R 1999 |
|
|
|
Letter
to Basic Lab Scientists
|
by Garrott Christoph, Ph.D. Laboratory of Chemical Physics, NIDDK |
The task put to me by the NIH Center for Information Technology (CIT) was daunting and even necessary. I was asked to help evaluate Y2K risk priorities for laboratory equipment and to help inform working scientists about their Y2k responsibilities. So, how do NIH scientists respond to the Y2K challenge?
A random survey of some friends and colleagues elicited the following responses:
![]() "Next January I will decide what is important and I will fix what is broken
then. I am not looking for problems now."
"Next January I will decide what is important and I will fix what is broken
then. I am not looking for problems now."
![]() "Just turn the damn computer dates back!"
"Just turn the damn computer dates back!"
![]() "Scientists are in the business of solving problems; they are not in the
business of looking for hypothetical problems."
"Scientists are in the business of solving problems; they are not in the
business of looking for hypothetical problems."
![]() "We are not clinical . . . don’t bother us."
"We are not clinical . . . don’t bother us."
![]() And my particular favorite, "So file dates are wrong—so what? Go
work on a real problem."
And my particular favorite, "So file dates are wrong—so what? Go
work on a real problem."
Add to this a matching tone of voice, an exaggerated eye rolling, and my own not unsympathetic preconceptions—well, you get the picture.
|
Y2K
Service Offer The Center for Information Technology is offering remediation services to the ICs to ensure that Unix systems achieve both Y2K compliance and an acceptable level of security. To take advantage of this offer, please contact Jim Sullivan at 6-7212. All NIH staff are
invited to attend Y2K Awareness Day, October 29 from 9:00 am to
12:00 noon in Masur Auditorium, Building 10. Panel discussions feature
representatives from NIH, PEPCO, financial institutions, local government,
and private sector organizations. Information booths will be set up to
help answer questions about how to prepare for Y2K both in the workplace
and at home. For more details about Y2K plans at the NIH, visit the web
site. |
But this is the reality. After an enormous effort by the NIH clearinghouse people, there remain 3,200 devices (about 25 percent of the NIH clearinghouse database) whose compliance status is either "NC" (not compliant), "CC" (conditionally compliant), or "NT" (not tested, meaning that their Y2K certification status is problematic or unknown). Equipment is old, some companies are unresponsive, others have gone out of business, new equipment is found, and complicated issues arise.
My assignment began by looking at the NIH Biomedical Clearinghouse with an eye toward composing a list of the most critical, most commonly used, and highest-impact equipment. CIT wanted a "Top Ten List of Vulnerable Equipment." It became clear that such a list was of limited value. Individual laboratories are quite different from one another; they are not rubber-stamped production centers. They have different equipment, different priorities, and different critical elements.
I searched the web for scare stories to help illustrate the potential seriousness of Y2K noncompliance and found examples of Y2K glitches in software and instrumentation control mechanisms that caused problems and sometimes even precipitated life-threatening crises. I found them in large integrated systems and stand-alone devices in clinical settings and in other disciplines. But I could not find them centered on scientific instrumentation. This does not necessarily mean that they do not happen—and it might mean that scientists just don’t post horror stories on the web.
The take-home lesson for me, after thinking about the issue and certifying the Y2K compliance in our own laboratories, was that there are surprisingly few Y2K scientific instrumentation questions in the basic science laboratory. This paucity makes it all the more easy and sensible to identify now those few pieces of critical or expensive equipment in your lab that might be adversely affected by Y2K and to avert the problems that otherwise will be encountered later.
The Y2K and Scientific Equipment
There seem to be three main prospective Y2K biomedical instrumentation concerns.
1. Computer-generated file dates can be wrong. This problem particularly affects computers that interface with scientific equipment and collect raw data. Many, indeed most, NIH laboratories use this kind of equipment. Problems arise most commonly when the instrumental software misinterprets data with erroneous time stamps. For example, a spectrometer might use an incorrect base-line correction spectrum when more recent—but date-corrupted—data files are available. There are also potentially serious repercussions if the principal investigator is pursuing a patentable idea that for legal reasons needs a correct time stamp.
2. Instrumentation software with built-in scheduled maintenance or other automatic operations can malfunction or shut down. Software-enforced maintenance schedules more commonly occur in clinical-care equipment than in straight scientific laboratory equipment. Basic scientists often view instrumentation as a child views a set of Lego blocks: as something to be taken apart and reassembled in ways that fit the needs of the current experiment. They tend not to purchase instrumentation with software-automated maintenance controls. However, instrumentation software may perform other routine operations predicated on date calculations from an internal calendar and so may pose Y2K problems.
3. Commercial and scientific databases that use date calculations are at risk. Homemade software, as it interfaces with scientific instrumentation, may perform improper date calculations leading to unintended errors. This scenario seems less of a biomedical instrumentation problem than a straight computer software problem. But if software either directly communicates with instrumentation or analyzes the results of data collected from scientific instrumentation, it should be evaluated for Y2K compliance. Particular attention should be given to any date calculations, date sorting, or data retrieval by date-logical test arguments.
The Bottom Line
Principal investigators should evaluate and upgrade the critical equipment now, or deal with the problems in January. It may be entirely appropriate to think about only a few of the most critical components in each laboratory now and put off the little stuff until later. A large fraction of the instrumentation with Y2K issues may have low impact or inconsequential effects. However, this is a case-by-case, PI-dependent decision. Walk around your lab and think about what equipment is critical to your own research. Call the manufacturers if necessary. Use the steps described in "A Process to Evaluate Y2K Compliance") and the NIH Biomedical Clearinghouse Database.
This web site provides a database of 12,000-plus scientific and biomedical instruments with detailed Y2K information for the scientific community. It has been carefully built, collated, and maintained by CIT personnel for over a year. It is an enormously valuable resource to the scientific and medical community here at NIH. It allows you to resolve immediately most instrumentation compliance questions.
But choices should be
made now, with a deliberate and clear understanding of the consequences. If
you put everything off until January, you will be in a boat with about 106 other
scientists worldwide, and all the rest of the technical, commercial, financial,
manufacturing, transportation, and other sectors of the economy whose strategy
is to wait until January. Each of these elements will be bombarding the industrial
sector, including the scientific instrumentation industry, for equipment and
software upgrade requests and for new equipment. They
will be swamped and there will be long backlogs. This may affect your research.
![]()
These comments are biased toward the perspective of physical and biochemical scientists; they do not reflect the interests of clinical research or practice. They derive from web searching and conversations with NIH scientists, particularly Charles Buckler, NIAID, Alec Eidsath, BEPS, and Dan Garrett, NIDDK.
|
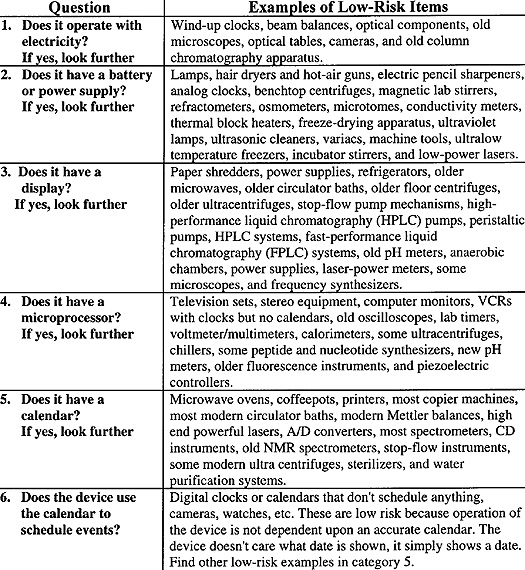
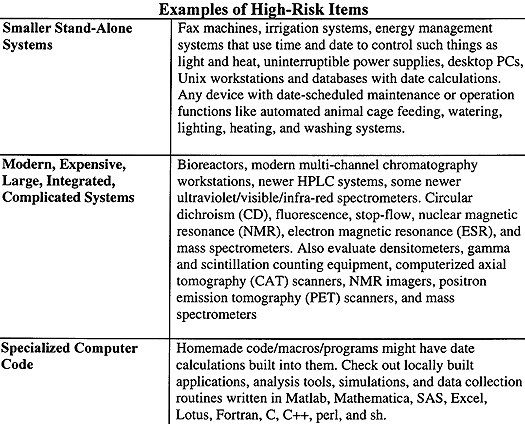
A Process To Evaluate Y2K Compliance While web searching for Y2K instrumentation problems, I found a useful "algorithmic prescription." It is used to identify instrumentation with Y2K issues. With it, you can cull a large laboratory-wide list of instrumentation to a short, manageable list of critical and problematic equipment. I used it in my laboratory. It worked, it was fast, and it strongly affirmed that I was asking the right questions. I modified it to include equipment examples found in many scientific laboratories. To identify embedded chip problems in stand-alone (noncomputer) electronic devices, answer these six questions. You might consider #1, and perhaps #2, gratuitous, but questions about such devices have arisen. |
||
 |
||
 |