
| T H E N I H C A T A L Y S T | M A Y - J U N E 1 9 9 8 |
|
MAMMALIAN PHEROMONE RECEPTION: OF MICE AND MEN? |
by
Nicholas Ryba, Ph.D, NIDR, and Roberto Tirindelli, Ph.D, Istituto di Fisologia Umana, Universita di Parma, Italy |
The recent report (1) that a short daily exposure of women to volatile compounds from sweat can significantly alter their menstrual cycles brought some new ripples of interest to mammalian pheromone research. This study reports opposing effects of sweat secretions gathered from groups of women at different stages of the menstrual cycle, providing some of the best evidence that pheromones-or perhaps a changing mixture of pheromones-may have significant effects on humans, as they do in other mammals. What the human pheromones are and where and how they act are unknown. It may be tempting-but premature-to try to fill in the unknowns in humans with speculations based on research in other animals.
In contrast to the very primitive understanding of human pheromones, a little more is understood about how rodents detect pheromones, thanks to significant progress over the past three years. Key steps were the cloning of two large families of putative pheromone receptors (2-5) in the labs of Richard Axel at Columbia and Linda Buck and Catherine Dulac, both at Harvard, and in our lab. In this article, we summarize some of this recent progress in research on rodent pheromone receptors, but strongly warn readers that this information may prove to have no bearing on Homo sapiens, for which, to date, there is no evidence that homologous receptors mediate pheromone responses. If there is one finding that we expect will carry over to humans from the rodent research, it would be that the pheromone receptor system is full of surprises.
Pheromones are used for intraspecific communication in organisms ranging from fungi to mammals. For example in yeast, specific peptides are secreted and result in a stereotyped mating response. Many insects have developed exquisitely sensitive systems that use volatile pheromones to attract and find mates. In most terrestrial vertebrates, pheromone effects have been well documented, and several unrelated molecules, including proteins, have been suggested as candidate mammalian pheromones. Many of these active molecules are contained in urine, sweat, or sexual or specialized gland secretions, and it is possible that specific mixtures rather than individual components are necessary to evoke the behavioral responses.

|
Pheromones are classified into two groups according to the timing or duration of their evoked responses. Releaser pheromones induce relatively fast behavioral responses, for example, sexual activity, parental care, or aggression. Primer pheromones elicit a sequence of slow physiological events that eventually influence specific aspects of reproduction. Lesion experiments in rodents suggest that the vomeronasal organ (VNO; see diagram), a chemosensory organ located at the base of the nasal septum that is distinct from the main olfactory epithelium (MOE), is responsible for several pheromone responses. These include: 1) Lee-Boot effect: the grouping of several female mice in a cage suppresses or modifies estrous; 2) Whitten effect: the induction of synchronized estrous by urinary cues of male mice in females with group-dependent estrous suppression; 3) Bruce effect: the physical presence or the urine of a male mouse of a different strain from the mouse to which a female has been recently mated prevents the implantation of embryos; and 4) Vandenbergh effect: puberty onset in female mice can be advanced by pheromones, most likely nonvolatile molecules contained in the urine of adult males.
The receptor cells in both the VNO and MOE are unusual neurons that turn over rapidly throughout life. Their axons take different routes: MOE neurons project to the olfactory bulb, whereas those of the VNO converge on the structurally distinct accessory olfactory bulb (AOB). The secondary projections of the MOE and VNO are also to separate areas of the brain. The principal connections of the olfactory bulb are toward the olfactory cortex. In contrast, the AOB projects to hypothalamic areas of the brain involved in hormonal and reproductive functions. However, during embryonic development, both the VNO and the MOE are derived from the same infolding of the olfactory placode, and the organization of the two neurosensory epithelia is similar. Given the similarities, it might be expected that both would make use of similar signaling mechanism.
Elegant work from several laboratories has provided a molecular explanation for the sense of smell. Early work from Randy Reed's group at Johns Hopkins established that olfactory reception probably involves a G-protein that controls the concentration of cAMP through a specific adenylate cyclase. A rise in cAMP in olfactory neurons directly gates a plasma-membrane ion channel and generates action potentials. Buck and Axel made use of the information that odorant receptors were linked to G-proteins to clone a vast family of about 1,000 distinct genes (6). The size of the family (perhaps 1% of all genes expressed in mammals) was completely unexpected. The first definitive evidence that any one of these mediates responses to a particular odorant was obtained this year (7). However, work principally from Axel's and Buck's laboratories demonstrating other properties of these receptors has already revealed much of the mechanism involved in the detection and encoding of the sense of smell (8). Discrimination of odors is possible because any olfactory neuron expresses only one of the repertoire of receptor proteins. Thus, there are effectively 1,000 different types of olfactory neuron, and the problem of discrimination is reduced to determining which neurons have been activated. In the epithelium, the distribution of receptors is essentially random (though in mammals, there are four distinct zones in which specific subsets of receptors are expressed). However, the axonal projections of olfactory neurons expressing specific receptor proteins converge. In fact, neurons expressing a given receptor have been shown to send axons to two specific glomeruli in each lobe of the olfactory bulb, and each glomerulus seems to receive innervation only from one type of sensory neuron. Moreover, the relative positions of the glomeruli where neurons expressing particular receptors converge are fixed. Thus, specific odorants produce a fixed pattern of activity in the olfactory bulb. How this pattern is set up and maintained as the neurons are continually replaced throughout life is quite relevant to under-standing the sense of smell and broader mechanisms of neural development.
As this work on odor detection evolved, our lab began exploring pheromone detection. The first surprise in the VNO was that most of the major components of the olfactory signaling system are absent: The olfactory G-protein and adenylate cyclase are not expressed at detectable concentrations; only a nonconducting subunit of the olfactory ion channel is present; and there are no close homologues of the odorant receptors. However, even though the molecular details are quite different, there still appear to be parallels between the ways the VNO detects pheromones and the MOE responds to smell.
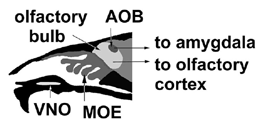
the location of the VNO and AOB relative to the MOE and olfactory bulb. |
|
Important evidence that VNO signaling is also likely to be G-protein-mediated was the finding that two distinct G-protein a-subunits are expressed at high concentrations in nonoverlapping populations of VNO neurons (9). However, attempts to clone receptors from the VNO based on homology to other G-protein-linked receptors were unsuccessful. The first breakthrough in identifying putative pheromone receptors was provided by a new, almost assumption-free approach that Dulac introduced (2). She argued that if the receptors were expressed with a pattern similar to odorant receptors, individual VNO neurons would express high amounts of one specific receptor and that this expression pattern would constitute the major difference between any two cells. Accordingly, she made libraries from individual VNO neurons, compared their cDNAs, and cloned a family of novel receptors (2). Perhaps the most important aspect of this work was the comparative single-cell approach, which points to differentially expressed genes and molecular expression patterns at a cellular level.
The sequences of the new family of VNO receptors revealed why the homology-based search for pheromone receptors had been unsuccessful. The receptors have no significant similarity to any known proteins, and the only clue that they might be G-protein-linked remains the fact that they contain seven stretches of sequence predicted to form membrane-spanning helices. The family is quite large: There appear to be 30-100 different genes with 50-90% sequence identity with one another (2). The size of the family immediately suggests that the neurons in the VNO are likely to be able to respond to a relatively wide range of ligands. Another important observation was that these receptors, now referred to as V1Rs, are only expressed in the apical neurons of the VNO. The neurons expressing the different G-proteins also are segregated, with those expressing Gai2 located in the apex and those expressing Gao located in the base of the VNO. This discovery opened the possibility that there could be a second family of receptors expressed in more basal G alpha o-containing neurons.
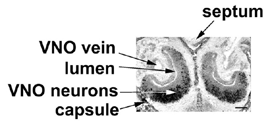
section of a neonatal rat. Probe stains mature VNO neurons; the bar is 250 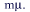 |
As it turns out, we had already identified a receptor from a second family of VNO receptors using essentially a nondirected approach (3). Dulac and Buck independently applied the single-cell comparison approach to clone other receptors from the same family (4, 5). The second family of VNO receptors, which we named V2Rs to distinguish them from the unrelated V1Rs, comprise as many as 100 genes that share homology with the parathyroid calcium-sensing receptor and the metabotropic glutamate receptors (3-5). The only similarity between V2Rs, odorant receptors, and V1Rs is structural: All three classes are predicted to have seven membrane-spanning helices. V2Rs are distinguished by their long N-terminal extracellular domains preceding the transmembrane helices. Ca2+ and glutamate appear to bind to this extracellular domain, and, as might be expected for receptors that bind multiple distinct ligands, they appear to be more divergent in this region than in their transmembrane domains. In contrast, V1Rs and odorant receptors exhibit most sequence divergence within the membrane-spanning helices, which are their presumptive ligand- binding regions.
Do the V1Rs and V2Rs function as pheromone receptors? Comparison of their expression patterns with those of the odorant receptors indicates that it is likely. First, just as odorant receptors are expressed in small subpopulations of MOE neurons, individual V1Rs and V2Rs are expressed in 0.5-3% of the VNO neurons. As is also the case for the odorant receptors, the subpopulations of neurons expressing any one receptor do not appear to overlap with those expressing other receptors. Moreover, the expression of V1Rs and V2Rs is restricted to sensory neurons in the VNO and they are not expressed elsewhere (2-5). These properties are consistent with both V1Rs' and V2Rs' functioning as pheromone receptors. Moving beyond such circumstantial evidence is harder, however: It took seven years to unambiguously demonstrate that one particular odorant receptor mediates a specific response (7). Therefore, even though V1Rs and V2Rs are good candidate pheromone receptors, it is likely that they will remain merely candidates for some time. As yet there are no clues to whether V1Rs and V2Rs bind distinct classes of ligand. However, given that compounds that have been reported to be vertebrate pheromones seem to be either small hydrophobic volatile molecules or proteins, it is conceivable that V1Rs bind one class of ligand and V2Rs the other. Clearly, if these families encode pheromone receptors, they must bind many active compounds, supporting the idea that mixtures of compounds elicit pheromone responses.
The presence of two families of unrelated receptors is, at present, the most surprising and intriguing aspect of signal transduction in the VNO. Why are three distinct families of G-protein receptors (with similar expression patterns but not sequences) required for chemosensory perception, and how is their expression controlled? The first question will probably best be answered by analyzing the evolution of the three families. The second question is the one that we are most keen to examine.
A particular problem encountered by all three groups that cloned the V2Rs was obtaining full-length clones. Despite evidence that the quality of mRNA we isolated was very good, most of the V2Rs were 5'-truncated mRNAs, appeared to contain introns, or lacked exons.
Surprisingly, some labs found that the V2R family of receptors is expressed in the olfactory epithelium of fish, which lack a VNO. Yet, in fish there is no problem obtaining full-length uninterrupted transcripts. This result raises the possibility that many of the mammalian counterparts are expressed pseudo-genes. Like higher vertebrates, fish respond to pheromones, and it is possible that this response is mediated through V2Rs. But unlike mammals, fish are able to smell amino acids. Given the similarity of V2Rs to the metabotropic glutamate receptors, it is conceivable that V2Rs are actually amino-acid or peptide receptors in fish. One possible evolutionary scenario is that as terrestrial vertebrates lost an unneeded ability to detect amino acids, there was no selective force against the mutation of many V2R genes, leaving only a few-adapted to recognizing pheromone cues-intact. While these observations lead some scientists to think many of the mammalian V2Rs encode expressed pseudogenes, other aspects of sequences suggest they encode proteins. Even if the majority are pseudo-genes, many are expressed at high concentrations in small subpopulations of VNO neurons.
One fascinating observation made by Dulac and a colleague is that one V2R displays some sexual dimorphism (4). In contrast, V1Rs and other V2Rs are expressed equally in female and male rodents. This finding suggests that the sexual dimorphism of most pheromone responses stems not from differences in signal reception but rather from differences in the way these signals are processed. Another aspect of the VNO revealed by these studies is its laminar organization: There are several distinct layers of neurons that express particular subsets of V2Rs. Moreover, the expression of some V2Rs extends even into what appears to be the Gai2 zone (3, 4) of the VNO. The Gai2 and Gao layers of the VNO project to distinct and contiguous regions of the AOB, suggesting that the receptor layers may also be preserved in the AOB. One major difference between these layers and the zones of the MOE is that the layers of the VNO develop during the first few postnatal weeks. In contrast, the MOE zones are defined early in embryonic development, before olfactory neurons make synaptic connections (10). This difference suggests that study of neural projections of specific VNO neurons may tell a rather different story from that of the MOE neurons and therefore may do more than just help explain pheromone signaling.
Do these molecular findings in rodents bear any relevance to the report of human pheromone responses? Perhaps the greatest surprise to scientists working in the field was that humans respond to pheromones at all. Humans have long been thought to possess only a vestigial accessory olfactory system, though some experts ranging from otolaryngologists to electrophysiologists now question this. Human genomic DNA encoding both classes of VNO receptors have also been isolated, but to date, all homologues are pseudogenes. Thus, the questions of where and how human pheromones work remain entirely open, as do the identity of the pheromones, whether humans possess a VNO, and whether it functions in the responses seen in Martha McClintock's headline findings.
References:
1. K. Stern and M.K. McClintock. "Regulation of ovulation by human pheromones." Nature 392, 177-179 (1998).
2. C. Dulac and R. Axel. "A novel family of genes encoding putative pheromone receptors in mammals." Cell 83, 195-206 (1995).
3. N.J.P. Ryba and R. Tirindelli. "A new multigene family of putative pheromone receptors." Neuron 19, 371-379 (1997).
4. G. Herrada and C. Dulac. "A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution." Cell 90, 763-773 (1997).
5. H. Matsunami and L.B. Buck. "A multigene family encoding a diverse array of putative pheromone receptors in mammals." Cell 90, 775-784 (1997).
6. L. Buck and R. Axel. "A novel multigene family may encode odorant receptors: a molecular basis for odor recognition." Cell 65, 175-187 (1991).
7. H. Zhao, L. Ivic, J.M. Otaki, M. Hashimoto, K. Mikoshiba, and S. Firestein. "Functional expression of a mammalian odorant receptor." Science 279, 237-42 (1998).
8. R. Axel. "The molecular logic of smell." Sci. Am. 273, 219-230 (1995).
9. M. Halpern, L.S. Shapiro, and C. Jia. "Differential localization of G proteins in the opossum vomeronasal system." Brain Res. 677, 157-161 (1995).
10. S.L. Sullivan, S. Bohm, K.J. Ressler, L.F. Horowitz, and L.B. Buck "Target independent pattern specification in the olfactory epithelium." Neuron 15, 779-789 (1995).