Abstract
The functioning of the nervous system is dependent on the network of connections among neurons that arise during development. This network forms when each neuron sends out an axon to its target cells during embryogenesis. One mechanism that contributes to guiding axons to their targets is long-range chemotropism: axons can be attracted by diffusible attractants, secreted by target cells and repelled by long-range chemorepellent substances, secreted by non-target cells, that create exclusion zones that the axons avoid. Our laboratory has been interested in identifying these long-range chemoattractants and repellents in order to determine their contribution to guidance in vivo and their mechanisms of action. We have focused in particular on axon guidance in the developing spinal cord, where spinal commissural axons are attracted to an intermediate target, the floor plate of the spinal cord, by a floor plate-derived attractant.
Through biochemical purification, we were able to identify a good candidate for the attractant, a novel 78-kDa protein we call netrin-1, as well as a closely related molecule, netrin-2. Netrin-1 is expressed by floor plate cells and can mimic the chemoattractant activity of floor plate cells in an in vitro assay. Direct evidence for the involvement of netrin-1 in guiding commissural axons along a dorsal-to-ventral circumferential trajectory in vivo was obtained when we found that these axons become misrouted in mice that have a mutation in the netrin-1 gene.
Remarkably, the netrins are vertebrate homologs of the UNC-6 gene product in the nematode Caenorhabditis elegans. Mutations in unc-6 impair circumferential migrations of axons and cells in the nematode. The finding that unc-6 is required for migrations in both a ventral and a dorsal direction has led to the hypothesis that UNC-6 may attract some axons while it repells others. We have found that this is true of netrin-1, because a population of axons that grow away from the floor plate, trochlear motor axons, are repelled by netrin-1. Thus, UNC-6 and the netrins define a highly conserved family of bifunctional axon-guidance molecules.
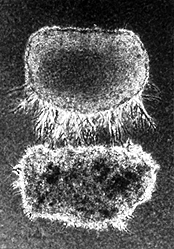
The chemotropic effect of netrin-1 on spinal commissural axons is shown in this in vitro growth experiment. An aggregate of COS cells secreting recombinant netrin-1 protein was placed slightly below tissue taken from the dorsal spinal cord of an embryonic rat. After incubation on a collagen matrix for 40 hours, the spinal commissural axons show abundant, but largely unidirectional outgrowth of bundles, or fassicles, of axons oriented toward the COS cell source of netrin-1. [Reprinted with permission from T.E. Kennedy, T. Serafini, J.R. de la Torre, M. Tessier-Lavigne, Cell 78, 425-35 (1994)] |
![[Brain]](brain.gif)