A window for every lab ... no more than a two-minute walk from research bench to patient's bedside ... . It may sound too good to be true, but those are only a few of the research-friendly features being contemplated for the streamlined new Clinical Research Center.
NIH Director Harold Varmus announced on Dec. 20 that the Zimmer Gunsul Frasca Partnership (ZGF) had bested five other architectural firms to become the design team for the new Clinical Center. The Portland, Ore., firm was the unanimous choice of a selection committee made up of NIH scientists, clinicians, and administrators, as well as design and planning experts.
 |
"What we have now is a concept. However, the actual building that ends up being built may be very different from the concept. No one should look at this as a final plan," says NCI Deputy Director for Clinical Affairs Gregory Curt, one of the researchers on the selection committee.
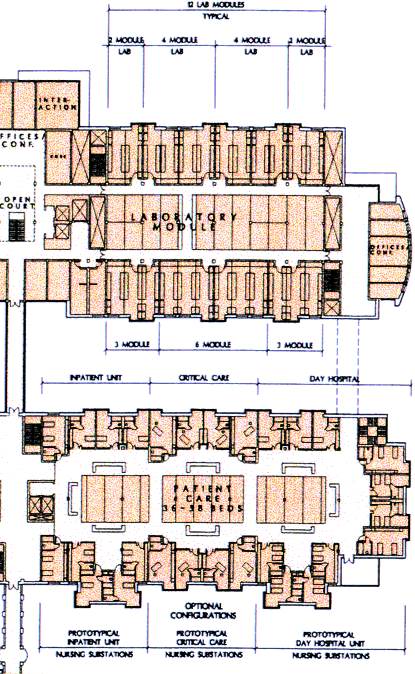
Another key question remains exactly how NIH will come up with the approximately $300 million needed to build the 850,000-square-foot facility, which will be attached to the north side of the existing Clinical Center. The year 2002 is the target opening date for the new Clinical Research Center, which will have 250 inpatient beds, down from the present 350.
"We gave very few specifications to the architects in advance. But one thing we did specify was the distance between labs and patient beds. ... We wanted them to be as close as possible," says Curt. "This was one of the most important things we could do to help translate basic research to the clinic."
Walter Armstrong, NIH's project director for the new Clinical Research Center, says ZGF was the only firm in the design finals that boasted experience in building both research labs and hospitals. The lab space that the firm has built or is planning to build includes facilities at the Fred Hutchinson Cancer Research Center in Seattle, the Johns Hopkins Medical School in Baltimore, the University of California system, and the Vollum Institute for Advanced Biomedical Research in Portland, Ore. Hospitals include a Veterans' Affairs medical center and a children's hospital, both in Portland.
Clinical Center Director John Gallin says, "The ZGF proposal is terrific because of its ability to accomodate the flexible demands that NIH has had and will have in the future. The design was also the least obtusive for the NIH campus and surrounding community. The laboratory design team that will join ZGF, Walls/Copenhagen of San Diego, is one of the world's best and we should end up with an attractive and very functional lab facility."
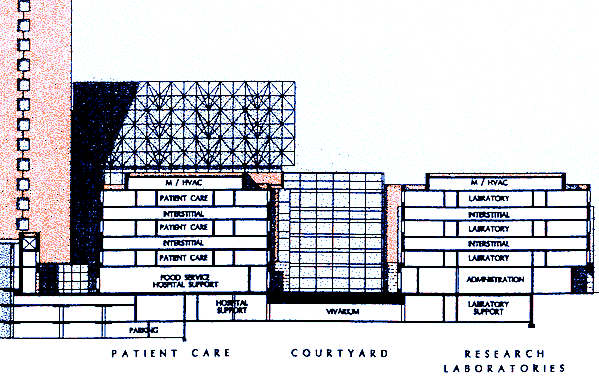
One innovative aspect of ZGF's vision of the Clinical Research Center is its "layer cake" design, which sandwiches floors of support systems between floors of labs or patient units. The support, or "interstitial," space would be used primarily to house mechanical, electrical, and venting systems for lab and hospital equipment, a design that allows renovations to be made much more quickly and at a substantially lower cost than in traditional buildings. Another major plus is that renovation work can be done with no disruption to activities in adjacent labs.
In the architect's initial concept, the basic lab in the Clinical Research Center would be the 11-foot by 33-foot module prescribed by NIH. These modules could either be enclosed or arranged in open lab units of up to 24 modules. Offices and conference rooms would be located at the end of each bank of labs, and an assortment of "small-group interaction areas," such as alcoves and balconies, would be arranged around a skylit open atrium connecting all lab levels with an open stairway. Because the dimensions of the lab modules are increments of the patient-room modules, the designers observed that it would be a simple matter to convert lab space to patient space and vice versa. Other features that lend themselves to interchangeability are the window spacing, which provides both researchers and patients with external views, and the flexible traffic patterns, which can be arranged to meet the varied security needs of a wide range of labs and patient areas.
 |
Before the architects begin to draw up any final designs, they plan to seek more input from both NIH and the surrounding community. "Researchers need to identify their scientific needs for the future and what [those needs] will require in terms of support," says NEI Scientific Director Robert Nussenblatt. As chief of NEI's Laboratory of Immunology, Nussenblatt is looking forward to using the new facility both for bench research and clinical trials.
ZGF, which plans to set up an office in the Bethesda area, will hold a series of public meetings and conduct detailed interviews with working researchers specified by the Medical Board. "The bottom line is that the design should be for the people who occupy the building. ... The time for researchers to make their views known is now, not after ground has been broken," says Armstrong. A display providing monthly updates on the design process will be set up in the main lobby of the Clinical Center. Researchers are also encouraged to e-mail their suggestions for the new facility to Clinical Center Director John Gallin (jg21z@nih.gov).