
NICHD's Joshua Zimmerberg, foreground, and Leonid Margolis assemble a NASA bioreactor.
Outer space may remain far beyond NIH's scientific reach for quite some time, but some intramural researchers are taking advantage of the next best thing-a chance to explore the earthbound applications of technology designed for the final frontier.
"The nice thing about working with NIH is that the rest of the scientific community looks to NIH for guidance, and if useful results are reported by NIH researchers, the technology starts to spread," says Stephen Davison, a biotech program manager with NASA's Microgravity Science and Applications Division in Washington.
NIDCD Director James B. Snow Jr., who was appointed NIH's representative to NASA's Life Sciences and Microgravity Advisory Committee last spring, agrees that NIH and NASA make compatible partners in many areas of biomedical research. "Research on Earth could benefit from the application or transfer of technologies specifically developed for space-related purposes, and research in space or space-like environments could improve knowledge of the normal function of human biologic systems on Earth," Snow told this year's American Institute of Aeronautics and Astronautics' Life Sciences and Space Medicine Conference in Houston.
Currently, two NIH labs - one at NICHD and one at NEI - are weighing the potential applications of NASA-developed technologies in basic biomedical research. Not only do the two projects tackle quite different scientific problems, but they also diverge in the nature of the devices being tested and in the formality of their arrangements with the federal space agency. NICHD's evaluation of NASA's bioreactor for three-dimensional tissue culture is a five- year, $4.8 million interagency agreement. On the other hand, NEI and NASA scientists' fledgling collaboration to explore the utility of a fiber-optic probe for cataract detection involves no exchange of funds and resembles traditional collaborations between NIH researchers and scientists in academia. What the two projects do have in common is that both arose through avenues that NIH researchers often overlook while scouting around for collaborative opportunities.
Until a friend in academia alerted him to one of NASA's bi-annual "Research Announcements," Joshua Zimmerberg, chief of NICHD's Laboratory of Theoretical and Physical Biology, didn't know that NIH scientists were even eligible for NASA grants, let alone that one of NASA's proposal requests dovetailed neatly with his lab's interests in three-dimensional tissue culture and the imaging of complex tissues [see box]. The request called for research projects that might facilitate the space-to- ground transfer of NASA's bioreactor, a fluid-filled, rotating-wall cylinder originally developed to allow cells to be cultured in liquid medium-despite the weightless conditions found in the space shuttle-and to protect the cell cultures aboard.
In the first year of the joint project, which got under way in August 1994, Zimmerberg's group, which includes three-dimensional-culturing expert Leonid Margolis, created a two-room core facility in Building 10 that is equipped and staffed to assist NIH and NASA researchers who want to use the bioreactor to address basic biomedical questions. For its part, NASA has moved one of its experienced technicians, Wendy Fitzgerald, from Houston to Bethesda to train scientists in the fine points of using the bioreactor. In addition, the collaborative agreement calls for Zimmerberg and his colleagues to use the NASA bioreactor to create tissue cultures for other NIH projects that require a higher level of cell organization than is available through standard monolayer or suspension culture. The NICHD lab is currently using the bioreactor to create a human lymphoid tissue model for AIDS research.

NICHD's Joshua Zimmerberg, foreground, and Leonid Margolis assemble
a NASA bioreactor.
The unique properties of the NASA bioreactor, which costs about $4,500 and is manufactured by Synthecon Inc. of Houston, aren't immediately apparent. Outwardly, the bioreactor looks virtually the same as a conventional "roller bottle" bulk-culturing device. However, NASA bioengineers have nested a second, slightly smaller rotating cylinder within the roller bottle to form a rotating wall of medium that keeps cultured cells or tissues in constant, gentle suspension rather than allowing them to slosh roughly about as they do in a standard roller bottle. The inner wall is semipermeable, to allow the free exchange of oxygen and carbon dioxide within the bioreactor.
Although NASA scientists designed the bioreactor to protect cells during space travel, they decided to pursue on-ground applications after discovering that cells cultured in the bioreactor's low-turbulence environment on Earth grew into three-dimensional masses that bore a greater structural and physiologic resemblance to natural tissues in vivo than did cells cultured by traditional methods.
Although the fledgling NIH-NASA bioreactor venture has yet to yield any publications, Zimmerberg says the most impressive results from the bioreactor to date fall into two general categories, engineering tissue-like structures from single cells and keeping tissue alive with its architecture intact in vitro.
The first set of achievements-making cells clump together and differentiate to form tissue-like spheroids of up to 3 mm in diameter-was accomplished by using the bioreactor exactly as NASA envisioned. "At this point in time ... if someone wants to grow spheroids for their research, this is the best environment in which to grow spheroids," Zimmerberg says. However, the NICHD team's recent success in maintaining 2- to 3-mm fragments of lymphoid tissue in the bioreactor for up to three weeks represents a more innovative application of the device. The ultimate goal of such efforts is to develop a uniform, controlled environment in which to study various aspects of human immunodeficiency virus (HIV) infection in human lymphoid tissue.
NICHD researchers have also learned a few things about the limitations of bioreactor technology. For example, Zimmerberg states that a "big drawback" of the bioreactor is its inability to run multiple controls in the same experiment. "In a 96-well plate, you can have 96 different conditions for cells growing, whereas in the bioreactor, it's just one homogeneous volume," he says. The lab is currently trying to get around that problem by wrapping different types of tissues in separate "envelopes" of agarose before they are placed in the bioreactor.
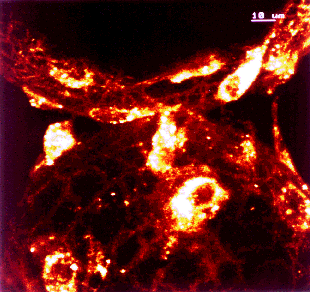
Confocal micrograph of bovine endothelial cells grown in the NASA bioreactor for
11 days. Cells attach to microcarriers and, due to the low shear forces in the
bioreactor, bridge them.
[Courtesy Leonid Margolis, NICHD.]
The rewards may be substantial for those NIH research teams whose research projects happen to mesh well with the bioreactor's strengths, according to Zimmerberg. For each of the three or four intramural projects deemed to be the best tests of the bioreactor's scientific potential, the NASA-NIH center will provide funding for a three-year postdoc position. That's on top of the training and support services of two technicians and a histologist funded by NASA.
To date, eight groups of intramural scientists have tried or are attempting to use the NASA bioreactor in their projects, with varying results. On the disappointing side, NHLBI's Maurice Burg says that although the bioreactor did keep renal medullary cells alive outside of the intact kidney somewhat longer than did other techniques, it did not keep those cells "lively enough long enough" for Burg's lab to perform its desired series of experiments. But Burg says his experience shouldn't dissuade others from testing the bioreactor: "They [the bioreactor center staff] were good people to work with- they work quickly and well." Like Burg, NICHD's David Klein found that the bioreactor did little to advance his research. "We failed to get encouraging results," says Klein, who had hoped the bioreactor environment would allow pineal gland cells to proliferate more freely and respond better to challenges from stimulants such as norepinephrine than they do in standard cell-culture conditions.
Others have had a bit more luck. Using a scaled-down version of the bioreactor, NIDDK's Gary and Liliane Striker report promising early results in culturing mesangial glomerular cells that more closely resemble those found within normal kidneys than cells grown by traditional techniques. "The initial bioreactor was too large," says Liliane Striker, who says the bioreactor center helped to modify the device for her group's experimental needs.
Although he only began testing the bioreactor a couple of months ago, NCI's William Stetler-Stevenson says that so far, he's seen nothing to make him stop exploring the use of the device in his studies of tumor-cell invasion. In their experiments, Stetler-Stevenson and his colleagues are attempting to grow tumor-like spheroids using a human melanoma cell line transfected with genes encoding tissue inhibitors of metalloproteinases (TIMPs), which suppress cell invasion by inhibiting matrix metalloproteinases. The researchers hope to use those spheroids to examine how varying the expression of TIMPs affects cell adhesion and migration in culture.
NIA's Steven Sollott is enlisting the bioreactor in his efforts to develop better in vitro models for studying cardiovascular disease. In experiments just recently started, Sollott's group is using bioreactors in attempts to culture vascular smooth muscle cells and endothelial cells in a manner that maintains the differentiation seen in the body. Under standard cell-culture techniques, such cells de-differentiate and behave differently than they do in vivo. Sollott and his colleagues are also co-culturing vascular smooth muscle cells and endothelial cells in the bioreactor in hopes that they may form structures similar to small blood vessels. "We're very excited about the potential of the bioreactor," Sollott says.
"It's hard to test out this technology simply by reading about it," says Zimmerberg, urging more intramural researchers to stop by and check out what's going on with the 18 or so NASA bioreactors the center has on hand. Although NASA naturally would like to see its brain child become a standard fixture in biomedical labs around the globe, Zimmerberg says that space agency officials have not interfered with his research and have made it clear they want the assessment of the bioreactor to be as objective as possible. "They were very excited about the idea of getting a completely unbiased evaluation," he says. "I've received nothing but support from the NASA people. ...There's no micromanagement."
Other institutions where NASA bioreactors are being or have been tested include Harvard University and the Massachusetts Institute of Technology in Cambridge, Mass., New England Deaconess Hospital in Boston, Huntington Medical Research Institutes in Pasadena, Calif., The Wistar Institute in Philadelphia, and the University of Texas Health Science Centers at San Antonio and Houston. But what NASA finds particularly appealing about the NIH center is the easy access it provides to a wide range of top-notch biomedical researchers. "What NIH brings to the table is a critical mass of scientific intellect that can be pulled together and applied to a given problem," says Neal Pellis, a project director for biotechnology at NASA's Johnson Space Center in Houston. "Within academia, you have to go to multiple universities stretched across the United States to get the same level of expertise, and you often cannot get the same level of cooperation."
NEI's interest in another NASA device can be traced to some fortuitous page flipping. Leon Ellwein, special adviser to the NEI director, says he was thumbing through TechReach, a newsletter put out by the Great Lakes Industrial Technology Center in Cleveland, when an article reprinted from the Federal Lab Consortium Newslink grabbed his attention. The topic? A small fiber-optic probe- originally designed to measure the growth of protein crystals in experiments aboard the space shuttle-now being tested as a "compact eye diagnostics device" by Rafat Ansari at NASA's Lewis Research Center in Cleveland.
"I decided to call Ansari out of the blue and ask him all about it [the device], and he was reasonably aggressive on following up," says Ellwein. During his first face-to-face meeting with Ansari in Bethesda, Ellwein realized that the NASA probe might prove useful in the animal studies of potential anti-cataract drugs being conducted in NEI's Laboratory of Mechanisms of Ocular Disease. He called the head of the lab, J. Samuel Zigler Jr., over for a spur-of-the-moment discussion with the NASA scientist.
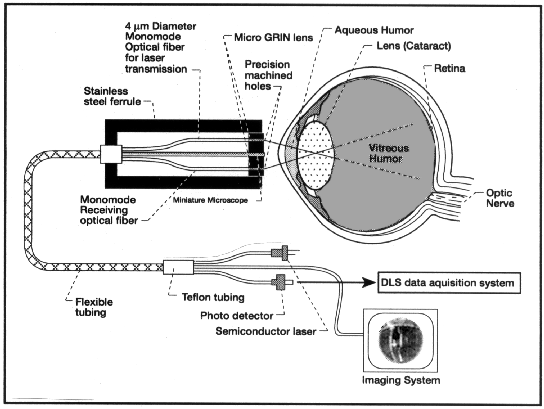
Schematic drawing of NASA's fiber-optic eye diagnostics device.
[Courtesy R. R. Ansari, Nasa Lewis Research Center, Cleveland.]
"Our realms of expertise are totally different - I know the biology and he [Ansari] knows the physics. But we may be able to find some common ground in the middle," says Zigler.
NASA's noninvasive probe, which is about the size of a pencil, consists of two optical fibers. The first fiber transmits a safe, low-power laser beam into the eye. The second fiber detects the laser's light as it is scattered within the eye and bounces back toward the probe. The resulting dynamic light-scattering data are processed by computer to generate a three-dimensional scan showing both the size and location of protein aggregates in the eye's lens. Some aggregation of proteins in the lens is part of the normal aging process, but when the clumps of proteins grow too large, they precipitate and create opaque, light- scattering centers. Such light scattering degrades the optical transparency of the lens, causing cataracts. Currently, surgical replacement of the clouded, natural lens with a plastic lens is the only treatment for cataracts, and more Medicare dollars-$3.4 billion in 1991- are used to pay for cataract sur-gery than for any other single procedure. There are no drugs on the market in the United States now to prevent or slow cataract formation.

J. Samuel Zigler Jr.
Photo Credit: Lorna Heartley
Unlike the clinical collaborations Ansari has established with the Wills Eye Hospital and Drexel University in Philadelphia, Zigler envisions using the NASA probe primarily in mouse models to screen for drugs that may halt or slow the progression of cataract formation. Preliminary results from mouse eye lenses that Zigler sent to Ansari indicate that the fiber probe can detect protein aggregations indicative of early-stage cataract formation. It remains to be seen whether the device can provide similar data when used on living animals. Currently, researchers must rely on slit-lamp examinations, which detect dark areas or shadows in normally homogenous tissue, to make relatively subjective assessments of changes within the animals' lenses. "If the difference between groups [in an anti-cataract-drug study] is small, it is difficult to establish anything with current methods," Zigler says. "This [NASA] device would be most valuable to us if it would provide an easy and reliable way to monitor changes in the lenses of the animals over time."
In addition to in vivo drug studies, Zigler says, he may try out the probe during in vitro experiments aimed at better characterizing how a molecular chaperone called alpha-crystallin acts to prevent the aggregation of denaturing proteins in the lens of healthy eyes. Ansari suggests that other possible biomedical applications of the NASA probe include detection of cholesterolosis in the eye's aqueous humor and of disorders affecting the vitreous humor.
To date, the bulk of the interactions between NIH and NASA on scientific projects has been conducted through extramural programs. NIDCD and NASA have established a ground-based center for balance and vestibular research at Northwestern University Medical School in Chicago. Through the Space Tissue Loss Program, NIAMS and NASA are funding extramural projects to conduct a series of experiments aboard the space shuttle that focus on the changes in bone and muscle cells during space flight. NINDS and NASA expect to fund up to seven extramural proposals for "Neurolab" experiments that will use the space shuttle as a unique environment in which to study neurological development and function. And the list goes on.
Among the NIH scientists who have served as consultants on extramural projects is NINDS's Daniel Alkon, who says the contacts he developed through such work paved the way for scientific exchanges between his lab and the Japanese space agency, NASDA. Alkon has provided the Japanese researchers with his simple-system model of learning and memory, the snail Hermissenda crassicornis. For their part, the Japanese scientists are working on developing an aquaculture environment in which the snails can be trained and maintained aboard a spacecraft. The researchers want to use the snail system to analyze the biophysical properties of memory and visual-vestibular associative learning under microgravity conditions. Some astronauts have experienced short-term memory deficits after space travel, but the basic mechanism underlying those deficits is unknown. Before the snails go up in space aboard a U.S. space shuttle-an event likely to occur within the next five years-Alkon says several Japanese scientists will probably come to NIH to study his lab's techniques. In exchange, Alkon hopes that he can share his Japanese colleagues' technological advances in high-resolution microscopy to obtain visual images of neuronal branches at the same time that electrophysiological recordings are being made.

Hermissenda crassicornis
[Courtesy Daniel Alkon and Carlos Collin, NINDS.]
In addition to the scientific incentives, the growing emphasis on efficiency in government may serve to promote greater interactions between NASA and NIH's intramural community. "Collaborations will occur whenever an opportunity to cooperate and coordinate will move the science forward faster and to a higher plane and when there will be cost savings to both agencies in accomplishing mutual goals," says Snow, adding, "Both NASA and NIH scientists possess enormous expertise and dedication."
To adapt John Donne's familiar phrase, no cell is an island. Although many types of cells can be grown through conventional culture techniques, such methods provide, at best, a pale imitation of the complex microenvironment that influences cell growth and activity within living tissue. Factors such as proximity to blood vessels and interactions with other types of cells don't come into play when cells are cultured under standard in vitro conditions. Recognizing a need to develop better ways of studying cells in a biomedically relevant context, Leonid Margolis' group at NICHD's Laboratory of Theoretical and Physical Biology has for the past seven years been working with methods that make it possible to culture and image three-dimensional blocks of tissue.
It has been known for decades that tiny chunks of tissue can be cultured for up to six weeks on collagen sponges floated in growth medium-a system used primarily for studying the tissue cells' invasion of the sponges. The NICHD group has gone one step further, however, by using the collagen-substrate system to study cell-cell interactions within tissues. Furthermore, Margolis, Joshua Zimmerberg, and their colleagues have taken advantage of a relatively recent technological advance in light microscopy-laser confocal fluorescence microscopy-to analyze the native architecture and dynamic processes within such tissue specimens, which are too thick for conventional microscopy. Compared with standard fluorescence microscopy, images formed using confocal fluorescence microscopy have much better spatial resolution because they have less out-of-focus light. Each plane of focus can also be conveniently stored in a computer database, thus facilitating quantitative analysis and allowing the reconstruction of three-dimensional images.
So far, Zimmerberg, Margolis, Boris Baibakov, and Svetlana Glushakova have used their three-dimensional histoculture and imaging techniques to track individual melanoma cells as they invade lung tissue. Using cubes cut from human tonsils, the researchers have also observed the fusion of healthy T cells with various types of human cells expressing the envelope glycoprotein of the human immunodeficiency virus (HIV). The lab has also used its culturing and imaging expertise to trace the innervation of the epithelial cells in rat tongue tissue by neuroblastoma and trigeminal ganglia cells and to observe differentiation of human breast tissue in vitro.
L.B. Margolis, S.E. Glushakova, B.A. Biabakov, C. Collin, and J. Zimmerberg. "Confocal microscopy of cells implanted into tissue blocks: cell migration in long-term histocultures." In Vitro Cell Dev. Biol. 31, 221-26 (1995).
L.B. Margolis, S.E. Glushakova, B.A. Baibakov, C. Collin, and J. Zimmerberg. "Syncytium formation in culture human lymphoid tissue: fusion of implanted HIV glycoprotein 120/41-expressing cells with native CD4+ cells." AIDS Res. Hum. Retroviruses 11, 697-704 (1995).
L. Margolis, B. Baibakov, C. Collin, and S.A. Simon. "Dye-coupling in three-dimensional histoculture of rat lingual epithelium." In Vitro Cell Dev. Biol. 31, 456-61 (1995).