IDENTIFYING SUBSTRATES IN THE BRAIN THAT UNDERLIE COCAINE
CRAVING
by Edythe D. London,
Ph.D., Steven Grant ,
Ph.D., and David Newlin,
Ph.D., NIDA
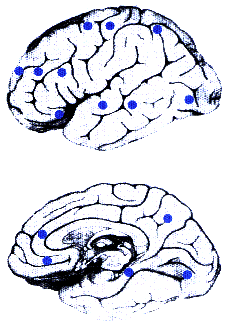
Schematic representation of the lateral (above) and medial
(below) aspects of the brain. Blue dots indicate brain
regions in which cocaine-related cues produced increases in glucose
metabolism compared with the effects of neutral cues. Affected
regions were located in prefrontal (superior, medial, and inferior
frontal gyri; pregenual cingulate; and medial and posterior orbitofrontal
gyri), temporal (superior and inferior temporal gyri), central
(pre- and postcentral gyri), parietal (angular gyrus), limbic
(parahippocampal gyrus), and occipital cortices (pre-cuneus, inferior
fusiform gyrus, and middle occipital gyrus).
The epidemic of cocaine abuse in the United States has underscored
the need for knowledge about the mechanisms by which cocaine alters
behavior. Such information is critical in the development of medications
to curb cocaine addiction. As part of this effort, we have applied
the [18F]fluorodeoxyglucose method to assess changes in regional
cerebral glucose metabolism, an index of local brain activity,
by positron emission tomography (PET) in cocaine abusers exposed
to cocaine-related cues. Our preliminary findings indicate that
the presentation of a videotape with cocaine-specific cues, exposure
to drug paraphernalia, and anticipation of cocaine self-administration
produce self-reports of cocaine craving, electroencephalographic
(EEG) arousal, and a characteristic metabolic pattern in the brain.
Exposure to cocaine cues is associated with stimulation of areas
of the occipital cortex, presumably because the cues are visual.
Also activated are areas of the prefrontal, temporal, parietal,
and limbic cortices. This work begins to define anatomical circuits
that are important in the response to environmental stimuli. These
circuits may link episodic memories of cocaine use to emotions
and to planning future drug taking.
Although the role of the mesolimbic dopaminergic pathways in supporting
reward and reinforcement from psychoactive drugs has dominated
contemporary drug-abuse research (1), it has become increasingly
evident that the pharmacological response to the drug per se is
only one factor that maintains compulsive drug use. Drug-induced
reward is a critical factor in the initiation or acquisition of
self-administration behavior, but hedonic responses to the drug
may not be critical to subsequent stages in addiction (2,3). In
fact, Tiffany (4) has suggested that continued drug self-administration
results in automatic cognitive and motoric habit patterns and
that relapse results from re-exposure to cues that elicit these
automatic patterns.
It is well-established that individuals who are drug-free can
relapse to abuse long after detoxification, and it is, therefore,
probable that chronic drug abuse produces persistent changes in
the nervous system that outlast the immediate effects on brain
reinforcement pathways. The presence of long-term changes suggests
that learning and memory are critical to the addictive process.
In this regard, almost 50 years ago, anecdotal reports of addicts
who left the Addiction Research Center of NIDA in Lexington, Ky.,
and then relapsed after returning to their old neighborhoods first
suggested that learning or conditioning factors may contribute
to recidivism (5).
There are two broad and conflicting theories regarding the responses
to drug-related environmental stimuli. Both propose that environmental
cues promote drug-seeking behavior via classical conditioning
mechanisms, but they differ with respect to the proposed relationship
between acute drug effects and the responses elicited by the cues.
The early proposals held that drug-related cues elicit an aversive
motivational state, which the addict attempts to escape by further
drug intake. The two major theories focusing on the aversive effects
of cues posited 1) that conditioned withdrawal phenomena produced
by drug-associated stimuli contribute to relapse (6), and 2) that
exposure to drug cues activates an opposing process that contributes
to tolerance (7). Either way, responses elicited by drug-related
cues would be in the opposite direction of responses produced
by the drug itself. For example, because cocaine increases arousal
and heart rate and produces euphoria, cocaine-related cues would
be predicted to decrease arousal and heart rate and to produce
dysphoria.
More recently, researchers have proposed that responses to cues
mimic aspects of the hedonic effects of drugs and, therefore,
that drug-seeking behavior is motivated by approach rather than
by avoidance (2,8,9). According to this view, cues activate reinforcement
systems in the brain and the addict seeks to maintain this activation
by engaging in drug-seeking behavior. These "approach"
theories propose that responses to drug-related cues contribute
to sensitization to drug effects because the conditioned response
is in the same direction as the acute effect of the drug (2,10,11).
In contrast to the "avoidance" predictions, the recent
hypotheses propose that cues related to cocaine use would increase
arousal and heart rate and would generate euphoria. Because there
is empirical evidence of both drug-like and drug-opposite responses
to drug-related cues (2,7,12,13), attempts have been made to reconcile
these conflicting theories. One suggestion is that the nature
of cue-elicited responses depends on the class of the drug taken.
For example, cues associated with opioid and sedative drugs have
been proposed to elicit drug-opposite and withdrawal-like responses,
whereas cues associated with stimulants, such as cocaine, elicit
drug-like responses (14). A second suggestion is that the direction
of the cue-elicited effect depends on the specific response measured.
Drug effects on afferent, or sensory, processes lead to drug-like
responses to cues, whereas drug effects on efferent, or motor,
processes produce drug-opposite cue-elicited responses (15).
Although conditioned responses to drug-related environmental stimuli
were first described by Pavlov (16), the brain systems involved
in drug conditioning have only recently been studied. Because
direct measurement of many brain systems requires invasive techniques,
these studies have been performed primarily in animals. Using
the induction of the immediate early gene c-fos as a marker for
neuronal activation, Fibiger and colleagues mapped the brain regions
in rats that respond to environmental cues associated with cocaine
administration (17). They found that drug-related cues increased
c-fos expression throughout the limbic system, including cingulate
cortex, amygdala, septum, and habenula. This distribution was
similar to that of the direct effect of cocaine on c-fos expression,
except that cocaine also increased c-fos expression in the caudate
and accumbens nuclei. The absence of increased c-fos expression
in any portion of the striatum is problematic for both "approach"
and "avoidance" models of drug conditioning because
they have emphasized the contribution of striatal areas to the
responses elicited by drug-related cues (9,18). Furthermore, because
dopaminergic input to these regions contributes to the reinforcing
and hedonic effects of drugs of abuse, the incentive motivational
theories propose that drug-related cues are reinforcing because
they also increase dopaminergic tone. It is known that cues associated
with delivery of biological reinforcers - such as food - increase
the firing of dopaminergic neurons in the brainstem and increase
dopamine release in the basal ganglia (2,19), but the evidence
for dopamine release in response to drug-related cues is contradictory
(2). Robinson and Berridge (2) recently suggested that although
the reinforcing effects of drugs are initially dependent on alterations
in dopaminergic tone, over time, other brain systems become dominant,
especially those associated with drug-seeking behavior elicited
by environmental stimuli.
Researchers have tested theories of drug conditioning in human
volunteers by presenting them with drug cues in the absence of
drug administration. Typically, the subjects view videotapes of
drug-taking behavior or conduct a self-administration ritual and
inject themselves with a placebo (14,20); this way, the conditioned
response to drug-associated stimuli is not obscured by the drug.
In studies of cocaine addicts, such experiments have shown that
drug-related cues lead subjects to report cocaine craving, accompanied
by reliable decreases in skin temperature and skin resistance,
increases in heart rate, and EEG arousal (21,22).
Although drug abusers may attribute their addiction to craving,
the extent to which craving drives drug-taking behavior may be
limited (13). For example, research volunteers with histories
of cocaine abuse reported less craving when treated with desipramine,
a proposed therapy for cocaine abuse; however, the volunteers
did not reduce self-administration of cocaine in the laboratory
(23). In another study of the efficacy of desipramine in the treatment
of cocaine abuse, decreases in self-reports of craving occurred
weeks after a decrement in cocaine use was observed (24). Nonetheless,
it is generally agreed that craving is "a subjective state
in humans that is associated with drug dependence" (25).
A current goal of our research on mechanisms of drug dependence
is to elucidate the biological determinants of craving for abused
drugs and the relationship, if any, between drug craving and drug
dependence.
To study the effects of cocaine-related cues on cerebral metabolism,
we paired PET measurements with psychophysiological assays and
self-reported subjective assessments in cocaine abusers during
two sessions. In one session, during the period when the radiotracer
([18F]fluorodeoxyglucose) was taken up after its intravenous injection,
subjects viewed a neutral videotape on arts and crafts. In the
second session, they were exposed to a cocaine-related stimulus
complex - a videotape of cocaine-related activity and paraphernalia,
the presence of actual paraphernalia, and a small amount of cocaine.
Analysis of data from the first nine subjects revealed increases
in self-reports of craving and overall arousal (decrease in EEG
power) during presentation of the cocaine-related stimuli compared
with the neutral session. Cerebral metabolic activation in response
to cocaine-related stimuli differed from activation during the
neutral session. The response also differed from the decrease
in brain activity observed previously in response to acute administration
of cocaine (26). The changes in response to cocaine-related stimuli
were "drug-opposite": the stimuli caused selective increases
in regional cerebral glucose metabolism, whereas acute cocaine
administration reduced glucose metabolism globally. Increases
due to cocaine-related cues, as distinct from neutral cues, were
observed throughout the prefrontal cortex, occipital cortex and
parahippocampal gyrus, with more restricted activations in the
parietal and temporal lobes and the pre- and postcentral gyri
(see figure). The metabolic changes produced by the cocaine-related
cues are consistent with the view that the response to drug-related
environmental stimuli may reflect activation of a distributed
neuroanatomical network, involving areas that mediate retrieval
of memories with affective components as well as those that may
participate in the planning of future drug self-administration.
These and other recent findings concerning the activation of brain
systems by exposure to cocaine cues raise interesting questions
for further study. The study of neural systems involved in highly
motivated behavior, such as addiction to drugs, may have important
implications for other forms of both adaptive and maladaptive
behaviors. In this regard, several medical disorders and risk
factors may entail acquired motivation to engage in other self-destructive
behaviors. Understanding cocaine craving may help us understand
craving for tobacco, alcohol, and opiates and the brain substrates
of normal and abnormal cravings for food in eating disorders.
Related questions concern paraphilic sexual motivation and preoccupation
with gambling. Moreover, it may be possible to determine whether
brain systems underlying acquired motivation for stimuli that
are not biologically relevant are similar to those of "normal"
motivation for biologically relevant stimuli such as food, water,
and sex.
Although our findings suggest that a specific anatomical network
is activated by cocaine-related cues and that this activation
is associated with cocaine craving, many questions remain unanswered.
To date, our studies in this area have focused on identifying
the brain regions important in responding to drug-related cues,
but the specific neurotransmitters mediating cocaine craving are
not known. The availability of radioligands for specific neurotransmitter
receptors and the development of tracers and mathematical models
for assessing neurotransmitter synthesis will facilitate further
work with PET scanning.
In clinical applications, it will be important to determine whether
the conditioned response to cocaine cues can be blocked pharmacologically,
either by agonists such as bromocriptine (27) or by antagonists
such as naltrexone (recently approved by the FDA for the treatment
of alcoholism). Key questions will be whether blocking craving
interferes with biologically necessary motivational systems and
whether suppressing craving will also prevent the actual taking
of abused drugs on the street.
Acknowledgment
We are indebted to our collaborators: Robert Phillips, Victor
Villemagne, Xiang Liu, Alane Kimes, and Carlo Contoreggi at NIDA
and Arthur Margolin at Yale University in New Haven, Conn. The
NIDA Brain Imaging Facility is supported by the Operating Budget
of the Intramural Research Program, NIDA, and the Office of National
Drug Control Policy.
References
1. E.D. London, S.J. Grant, M.J. Morgan, and S.R. Zukin. "Neurobiology
of drug abuse." In: Neuropsychiatry: A Comprehensive
Textbook. B.S. Fogel, ed. (in press).
2. T.E. Robinson and K.C. Berridge. "The neural basis of
drug craving: an incentive-sensitization theory of addiction."
Brain Res. Rev. 18, 247 - 91 (1993).
3. J.S. Altman, B.J. Everitt, M. Fischman, S. Glautier, A. Markou,
D. Nutt, et. al. "The biological, social and clinical bases
of drug addiction." Psychopharmacology (Berl)
(in press).
4. S.T. Tiffany. "A cognitive model of drug urges and drug-use
behavior: role of automatic and nonautomatic processes."
Psychol. Rev. 97, 147 - 68 (1990).
5. A. Wikler. "Conditioning factors in opiate addiction and
relapse." In: Narcotics. D.M. Wilner and G.G. Kassebaum,
eds. New York: McGraw Hill, pp. 85 - 100 (1948).
6. A. Wikler. "Dynamics of drug dependence: implications
of a conditioning theory for research and treatment."
Arch. Gen. Psychiatry 28, 611 - 16 (1973).
7. S. Siegel. "Classical conditioning, drug tolerance, and
drug dependence." In: Research Advances in Alcohol
and Drug Problems, Volume 7. R.G. Smart, F.B. Glaser, Y.
Israel, H. Kalant, R.E. Popham, and W. Schmidt, eds. New York:
Plenum, pp. 207 - 44 (1983).
8. J. Stewart, H. de Wit, and R. Eikelboom. "Role of unconditioned
and conditioned drug effects in the self-administration of opiates
and stimulants." Psychol. Rev. 91,
251 - 68 (1984).
9. J.P. Vincent, B. Kartalovski, P. Geneste, J.M. Kamenka, and
M. Lazdunski. "Interaction of phencyclidine ("angel
dust") with a specific receptor in rat brain membranes."
Proc. Natl. Acad. Sci. USA 76, 4678 - 82 (1979).
10. P.W. Kalivas, B.A. Sorg, and M.S. Hooks. "The pharmacology
and neural circuitry of sensitization to psychostimulants."
Behav. Pharmacol. 4, 315 - 34 (1993).
11. R.M. Post and S.R.B. Weiss. "Sensitization and kindling:
implications for the evolution of psychiatric symptomatology."
In: Sensitization in the Nervous System. P.W.
Kalivas and C.D. Barnes, eds. Caldwell, NJ.: Telford, pp. 257
- 93 (1993).
12. R.S. Niaura, D.J. Rohsenow, J.A. Binkoff, P.M. Monti, M. Pedraza,
and D.B. Abrams. "Relevance of cue reactivity to understanding
alcohol and smoking relapse." J. Abnorm. Psychol.
97, 133 - 52 (1988).
13. L.O. Bauer. "Psychobiology of craving." In:
Substance Abuse. A Comprehensive Textbook. J.H.
Lowinson, P. Ruiz, R.B. Millman, and J.G. Langrod, eds. Baltimore:
Williams & Wilkins, pp. 51 - 5 (1992).
14. A.R., Childress, R. Ehrman, D.J. Rohsenow, S.J. Robbins, and
C.P. O'Brien. "Classically conditioned factors in drug dependence."
In: Substance Abuse. A Comprehensive Textbook.. J.H.
Lowinson, P. Ruiz, R.B. Millman, and J.G. Langrod, eds. Baltimore:
Williams & Wilkins, pp. 56 - 69 (1992).
15. R. Eikelboom and J. Stewart. "Conditioning of drug-induced
physiological responses." Psychol. Rev.
89, 507 - 28 (1982).
16. I.P. Pavlov. In: Conditioned Reflexes; An Investigation
of the Physiological Activity of the Cerebral Cortex. London:
Oxford University Press, (1927)
17. E.E. Brown, G.S. Robertson, and H.C. Fibiger. "Evidence
for conditioned neuronal activation following exposure to a cocaine-paired
environment: role of forebrain limbic structures." J.
Neurosci. 12, 4112 - 21 (1992).
18. G.F. Koob, L. Stinus, M. Le Moal, and F.E. Bloom. "Opponent
process theory of motivation: neurobiological evidence from studies
of opiate dependence." Neurosci. Biobehav. Res.
13, 135 - 40 (1989).
19. W. Schultz. "Activity of dopamine neurons in the behaving
primate." Semin. Neurosci. 4,
129 - 38 (1992).
20. C.P. O'Brien, R.N. Ehrman, and J.W. Ternes. "Classical
conditioning in human opioid dependence." In: Behavioral
Analysis of Drug Dependence. S. Goldberg and I.P. Stolerman,
eds. Orlando, FL.: Academic Press, pp. 329 - 56 (1986).
21. R.N. Ehrman, S.J. Robbins, A.R. Childress, and C.P. O'Brien.
"Conditioned responses to cocaine-related stimuli in cocaine
abuse patients." Psychopharmacology (Berl) 107,
523 - 9 (1992).
22. L.O. Bauer and H.R. Kranzler. "Electroencephalographic
activity and mood in cocaine-dependent outpatients: effects of
cocaine cue exposure." Biol. Psychiatry 36,
189 - 97 (1994).
23. M.W. Fischman, R.W. Foltin, G. Nestadt, and G.D. Pearlson.
"Effects of desipramine maintenance on cocaine self-administration
by humans." J. Pharmacol. Exp. Ther.
253, 760 - 70 (1990).
24. F.H. Gawin, H.D. Kleber, R. Byck, B.J. Rounsaville, T.R. Kosten,
P.I. Jatlow, et al. "Desipramine facilitation of initial
cocaine abstinence." Arch. Gen. Psychiatry 46,
117 - 21 (1989).
25. R.W. Pickens and C.E. Johanson. "Craving: consensus of
status and agenda for future research." Drug Alcohol
Depend. 30, 127 - 31 (1992).
26. E.D. London, N.G. Cascella, D.F. Wong, R.L. Phillips, R.F.
Dannals, J.M. Links, et. al. "Cocaine-induced reduction of
glucose utilization in human brain. A study using positron emission
tomography and [fluorine 18]fluorodeoxyglucose." Arch.
Gen. Psychiatry 4, 567 - 74 (1990).
27. H.R. Kranzler and L.O. Bauer. "Bromocriptine and cocaine
cue reactivity in cocaine-dependent patients." Br.
J. Addict. 87, 1537 - 48 (1992).
Table of Contents
