by Kenneth Korach, Chief, Receptor Biology Section, Laboratory of Reproductive and Developmental Toxicology, NIEHS
Since estrogen's discovery in the 1920s, scientists have believed that the hormone plays a crucial role in embryonic, fetal, and adult development, and influences female secondary sexual characteristics, reproductive cycle, fertility, and maintenance of pregnancy (1). In several target sites in the body -- most notably, the reproductive tract, breasts, and neuroendocrine sites -- estrogen's action is central to normal adult physiology and function (2). The dramatic lowering of estrogen concentrations that occurs during menopause has been implicated as one factor in osteoporosis and cardiovascular disease, but these effects are poorly understood, and it is unclear whether estrogen elicits a direct tissue action or indirect effects involving other regulators or signaling systems. Recently, in collaboration with the Developmental Endocrine Pharmacology Group at NIEHS, we found that other cellular signaling systems play a role in the mechanism of estrogen stimulation in reproductive tract tissue (3). The role of estrogen in men is even less well understood.

The demonstrated importance of estrogen in development and function, combined with uncertainties regarding its role, mechanisms, and sites of normal and pathological action, made knocking out the estrogen receptor (ER) gene to disrupt the expression of functional ER protein a highly desirable experimental goal, but also an endeavor that was unlikely to be successful or result in viable animals (4). However, we reasoned that if the knockout was lethal, we could finally determine the stage and possible sites during development at which estrogen becomes critical.
Clinical evidence only increased our suspicion that an ER knockout would be futile, because no known conditions of estrogen insensitivity or ER gene mutations had been reported. In contrast, conditions of resistance to other hormones, due to defects in other members of the hormone receptor gene family have been reported. Androgen insensitivity caused by disruptive mutations of the androgen-receptor protein results in abnormal male sexual differentiation and development (5). Thyroid (6) and glucocorticoid (7) resistance are other examples of clinical endocrine conditions that can result from receptor gene defects. Scientists attributed the lack of reported cases of estrogen insensitivity in humans and experimental animals to lethality during development (1) or to effects on embryo implantation. Blastocysts and two-cell embryos express estrogen receptor mRNA, supporting the possibility of an early developmental role (8).
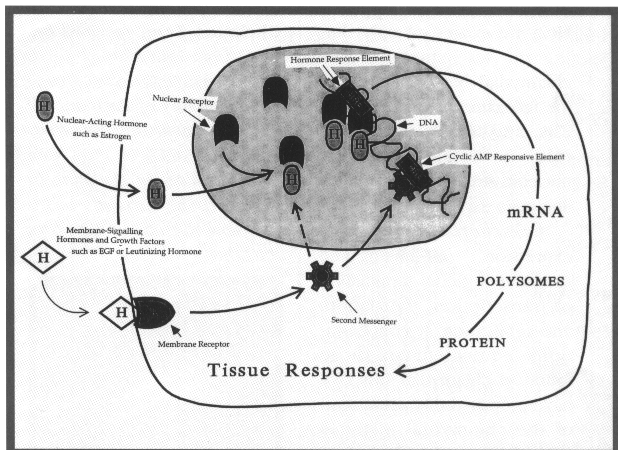
Estrogens trigger their broad array of physiological responses, including tissue differentiation, growth, protein synthesis, and secretion (9, 10), by binding to a nuclear-receptor protein. Activation of the receptor induces tissue- and organ-specific responses to the hormone. The estrogen receptor is a ligand-inducible transcription factor that modulates target genes after it binds estrogen. Past findings had indicated that estrogen steroid hormones are required for tissue effects mediated by the receptor, but surprisingly, we demonstrated that specific growth factors, such as epidermal growth factor, could mimic estrogen in stimulating some biological responses. Mechanistically, this growth-factor action appears to operate via the estrogen receptor, providing a means of multiple signaling that converges and induces a tissue-specific response. Development of an animal model in which the two signaling systems were uncoupled--for instance, by eliminating a functional estrogen-receptor system--would allow the evaluation of the role of the dual signaling systems in physiological regulation.
Surprise One: A Viable Mouse
Defying our own skepticism, in 1990, we established a collaboration with Oliver Smithies' laboratory at the University of North Carolina in Chapel Hill and launched an attempt to produce a mouse homozygous for disrupted function of the estrogen receptor. We succeeded by inserting a sequence encoding neomycin resistance into exon 2 of the mouse estrogen-receptor gene. The neomycin insert includes a premature stop codon and polyadenylation sequences that inhibit proper transcription and translation of the ER gene, thereby functionally inhibiting its expression. Successful targeting of the sequence by homologous recombinations occurred in only two of 1,800 clones of embryonic stem cells in which we attempted to disrupt the ER gene. Several chimeric mice bearing the disrupted gene were produced, including one in which the mutation was transmitted through the germ line. Mating of this chimera produced heterozygous mice of both sexes. These mice, bearing one copy of the wild-type ER gene and one copy of the inactivated ER gene, were screened by Southern and polymerase chain reaction analyses to ensure heterozygosity. These heterozygotes were fertile and exhibited no remarkable phenotype associated with disruption of one ER gene. Crosses of the heterozygotes resulted in normal litter sizes of live offspring with a traditional Mendelian distribution of genotypes. An even sex ratio was seen in the mice homozygous for ER gene disruption, indicating that sex determination is not influenced by the absence of a functional estrogen receptor. Most importantly, these mice provided the first evidence that absence of an active estrogen receptor is not lethal. Attempts to breed the homozygous ER-negative mice showed they were infertile.
We are curently analyzing the tissues of heterozygotic- and homozgotic-recessive animals to check for alterations in phenotype associated with inactivation of the estrogen receptor. Characterizing the transgenic line (ERKO), we are now analyzing the molecular effects of different types of hormonally active compounds, such as diethylstilbestrol (DES) and Tamoxifen, to see whether any other, as-yet-undescribed proteins or receptors may be present that can mediate estrogenic activity.
The ERKO females may be the first physiological model for critically testing the role and action of estrogen in the ovary. Initial analyses of the first recessive females have shown that they contain reproductive tract structures but lack any of the uterine responses to estrogen treatment seen in animals with ERs. ERKO females also have hemorrhagic cystic ovaries, suggesting over-stimulation by gonadotropins due to the lack of a functional negative-feedback mechanism. Ovarian histology shows no functional corpora lutea, even though the granulosa and thecal cells, which normally surround developing ova, are present. Folliculogenesis proceeds through primary and secondary stages but stops short of a terminal stage, with no ovulatory follicles present. Further analyses of the ovaries are being performed to evaluate biochemical indices of response to exogenous stimulants.
One of the most surprising findings was that ER-recessive male mice are infertile but appear to have anatomically normal male accessory sex organs. Histological analysis indicates that sperm are present in the testis and epididymis, but the sperm count is less than 10 percent of that in normal mice. ER-recessive male mice should be useful for evaluating the role of the ER in male reproductive biology.
Other observations on the ERKO mice are suggesting additional experiments. For example, adult female mice lacking the ER have undeveloped mammary glands. We are now attempting to cross an onco-mouse having an increased incidence of mammary cancer with ERKO heterozygotes to test whether the estrogen receptor is necessary for the development of breast cancer. Of particular interest to our own studies was the observation that the bone density of ERKO males and females is 20 - 25% lower than it is in wild-type mice. This suggests a direct role for estrogen-receptor action in bone physiology.
Once the ER-null mouse is characterized, it should be useful in understanding whether the effects of environmental chemicals associated with estrogenic-like effects operate through the classical estrogen-receptor signaling pathway. The mice could also be used to assess the activity of various drug preparations for possible estrogen-like activities. Similarly, groups of ERKO mice will be treated with DES to determine whether the same reproductive tract and other target-tissue cancers develop in these mice as develop in humans and wild-type animals.
ERKO transgenics are also being used as the background strain on which to reintroduce mutant estrogen-receptor protein (e.g., TAF-1 or TAF-2 deletion mutants) by classical gene-transfer technology. Previously, analyses of the expression and function of these mutant receptors could only be done by transfection studies in vitro. Now, animals can be produced that express only the mutant receptors, permitting analysis of tissue and gene regulatory specificity of the mutant receptor under physiological conditions in vivo.
Reintroduction of ER protein using a tissue or cell-type-specific promoter can test for rescue of the recessive phenotypes.
Surprise Two: A Clinical Link
After we discovered that disruption of the estrogen-receptor gene was not lethal in our mice, we became curious about the absence of reports of parallel human syndromes or gene mutations. It has been pointed out that because of their genetic backgrounds, some experimental knockout mice may not totally reflect what would be expected for comparable conditions in humans (11). Thus, although our finding was exciting from an experimental standpoint, we were doubtful about its application and relevance to human physiology.
Our doubts were dispelled late last year when we were contacted by a colleague at the Children's Hospital in Cincinnati about a 28-year-old fully masculinized male patient who presented at the clinic for genu valgum (knock-knees) and upon examination was found to have unclosed epiphysis. The patient was insensitive to high-dose estrogen treatment, showing none of the expected side effects, such as gynecomastia. In light of our finding that ER gene disruption was not lethal, the attending physician considered the possibility that the patient had some inactivation of estrogen-receptor function and sent us blood samples for molecular genetic analyses.
Our analyses demonstrated that the patient, the offspring of a first-cousins marriage, was homozygous for a mutation in exon 2 of the estrogen receptor gene. The mutation resulted in the creation of a premature stop codon, producing truncation of expression of a functional estrogen-receptor protein (12). This is the first example in humans of a loss-of-function mutation in the estrogen-receptor gene producing an estrogen-insensitivity syndrome. In addition to nonclosure of the epiphysis, the patient has dramatically low bone density, a symptom similar to that observed in the ERKO mice. This patient has raised our confidence that the ERKO mice may be an acceptable model for a variety of human estrogen responses and accompanying mechanisms.
It is especially satisfying that this high-risk project, which initially seemed to have little likelihood of success, has yielded an animal model containing no functional estrogen receptor. This model is now giving us the ability and opportunity to evaluate the role of estrogen-hormone action in a variety of tissues at different developmental stages. Estrogen's importance in mediating physiological responsiveness and its role in cancer and other pathological conditions may finally be determined. We now hope to see this experimental system rapidly applied to answer clinical questions regarding osteoporosis, cardiovascular biology, and breast, endometrial, and ovarian cancers.
References
1. F.W. George and J.D. Wilson. "Sex determination and differentiation." In: The Physiology of Reproduction, E. Knobil, ed. New York: Raven Press, 1988: 2 - 27.
2. J.D. Wilson, D.W. Foster eds.Textbook of Endocrinology, Philadelphia: W.B. Saunders, 1985.
3. D.M. Ignar-Trowbridge, K.G. Nelson, M.C. Bidwell, S.W. Curtis, T.F. Washburn, J.A. McLachlan, et al. "Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor." Proc. Natl. Acad. Sci. USA 89, 4658 - 62 (1992).
4. D.B. Lubahn, J.S. Moyer, T.S. Golding, J.F. Couse, K.S. Korach, and C. Smithies. "Alternation of reproductive function but not pronatal sexual development after insertional disruption of the mouse estrogen receptor gene." Proc. Natl. Acad. Sci. USA 90, 11162 - 6 (1993).
5. J.D. Wilson, J.E. Griffin, M. Leshin, and P.C. MacDonald. In: The Metabolic Basis of Inherited Diseases J. B. Stanbury, et al eds, New York: McGraw-Hill, 1993: 1001 - 26.
6. M.T. McDermott and E.C. Ridgeway. "Thyroid hormone resistance syndromes." Am. J. Med. 94 424 - 32 (1993).
7. G.P. Chrousos, S.D. Detera-Wadleigh, and M. Karl. "Syndromes of glucocorticoid resistance." Annu. Int. Med. 119, 1113 - 24 (1993).
8. Q. Hou and J. Gorski. "Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development." Proc. Natl. Acad. Sci. USA 90, 9460 - 4 (1993).
9. K.S. Korach. "Selected biochemical actions of ovarian hormones. Target organ toxcity: Endocrine System Conference." Environ. Health Perspect. 38, 39 - 45 (1981).
10. J. Gorski and F. Gammon. "Current models of steroid hormone action: a critique." Annu. Rev. Physiol. 38, 425 - 50 (1976).
11. R.M. Winter. "The importance and methods of using animal models to study disease." Growth Genet. Hormone 7, 6 - 8 (1993).
12. E.P. Smith, J. Boyd, G.R. Grank, H. Takahashi, R.M. Cohen, B. Specker, et al. "Estrogen insensitivity syndrome in an adult man caused by a homozygous nonsense mutation of the estrogen receptor gene." New Engl. J. Med. (submitted).