| T H E N I H C A T A L Y S T | M A R C H – A P R I L 2008 |
|
|
|
The
problem, policy, history & fix
|
by Christopher Wanjek |
 |
|
from
the Cell Line Authentication Global Awareness Initiative website |
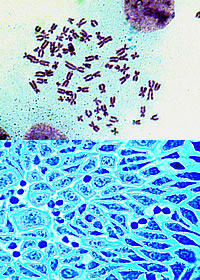
If only mouse cells had ears, whiskers, and a tail. These would make it much easier to identify the type of cross-contamination that for decades has plagued laboratories, invalidated years of research, sidetracked careers, and possibly squandered millions of research dollars.
In well-documented cases that highlight both the range and ubiquity of the situation, guinea pig cells have turned out to be mouse cells, ovarian cancer cells have been disguised as breast cancer cells, and, in extreme cases, some cell lines have been unidentifiable.
NIH is not immune, neither in the research performed here nor in the products produced. Many cell lines from reputable sources are accidentally mischaracterized or masquerading as another kind of cell unbeknownst to the supplier or user.
At least three lines in the respected and much utilized NCI-60 cancer cell lines are mischaracterized—such as MCF-7/AdrR, which was once thought to be a daughter of the breast cancer line MCF-7 but in reality is an ovarian cancer cell line.
More than 15 percent of cell linesmight be cross-contaminated or improperly characterized, according to peer-reviewed papers published in recent years. The issue has been addressed in the pages of Science, Nature, and other prominent journals and has prompted position statements from professional organizations. Yet the problem lumbers on largely unabated.
Cell-line cross-contamination commonly refers to when a foreign cell, usually mammalian, is introduced inadvertently into a culture of living cells and becomes a resident of that culture, either coexisting or entirely replacing the original line. Contamination from bacteria, yeast, and other invaders is also problematic, but for the most part researchers know how to identify or prevent this kind of contamination.
"We are fundamentally in agreement that misidentified and contaminated cultures constitute a serious, ongoing problem," said Michael Gottesman, deputy director for intramural research. "What is debated is how to best eliminate it."
The NIH intramural and extramural programs have stopped short of mandating cell-line authentication as a requirement for funding. The reasoning is that authentication methods can be specific and are continuously evolving, making it impractical for NIH to require application of particular methods, as relayed in an NIH policy notice issued on November 27, 2007, posted here.
For now, in lieu of a consensus statement, Gottesman and Norka Ruiz Bravo, deputy director for extramural research, advocate awareness and diligence. They would like to see the cell-line cross-contamination and misidentification addressed in the peer-review process, while educating scientists on their responsibilities to characterize their model systems.
The NIH policy notice, entitled "Notice Regarding Authentication of Cultured Cell Lines," was in response to an open letter sent to DHHS Secretary Michael Leavitt, dated July 7, 2007, by Roland Nardone, professor emeritus at The Catholic University of America in Washington, D.C., and co-signed by 18 other cell biology experts from the United States and United Kingdom.
This letter, posted here, calls for a "no authentication/no grant" approach by NIH, as well as for journals to require proof of authentication as a requirement for peer-review publication.
"The NIH notice strongly suggests that peer reviewers of grants and manuscripts could be the key to compliance," said Nardone. "I do not subscribe to that view because the pool of reviewers lacks the background knowledge and it will be some time—if ever—before that hole in the fence gets closed."
He noted that the FDA has a requirement for cell-line authentication as a condition for drug approval. NIH, he said, has a different culture and feels such an approach would be dogmatic.
NIH Director Elias Zerhouni has since tasked Nardone with bringing together a diverse group of scientists, scientific societies, grant reviewers, and scientific publishers to establish a consensus policy.
Nardone leads a campaign started in the 1960s by Walter Nelson-Rees, a biologist then at the University of California, Berkeley, who discovered that more than 40 individual cell lines had been overtaken by HeLa cells, cervical cancer cells cultured from a Baltimore woman’s tumor in the early 1950s.
Nelson-Rees alienated some scientists with his series of papers in the early 1970s in Science, naming labs and false cell lines and calling on the community to re-evaluate research based on these cell lines.
"The problem was reported to be widespread, so much so that it encouraged disbelief," Nardone said. Yet the problem has not gone away and may have gotten worse, he observed
"Cell lines shown in 1966 to be misidentified or cross-contaminated are still being used as if they were the real thing," Nardone said, referring to a publications database search he conducted with colleagues.
To minimize the risk of contamination or misidentification, Nardone offers four points of action, which the NIH Office of Intramural Research also endorses:
| BACKGROUNDERS |
| The NIH intramural community can learn more about cell-line contamination and safe laboratory practices in two courses offered by Bio-Trac*, the Biotechnology Training Courses. These are "TRAC 36: Cell Line Identification & Authentication" and "TRAC 7: Animal and Human Cell Culture: Method and Applications." Online information can be found at Cell Line Authentication Global Awareness Initiative. An unofficial list of contaminated cell lines can be found here. *Ed.
note: Bio-Trac is offered through FAES
under a contract with R/M Nardone Associates, Inc., a successor to a company
founded by Roland Nardone.
|
![]() Primary cultures and finite cell lines (for example, noncancer) should be sufficiently
characterized to confirm their source, such as species, tissue type, or the
individual for human cells, if relevant; even primary cultures can become cross-contaminated.
Primary cultures and finite cell lines (for example, noncancer) should be sufficiently
characterized to confirm their source, such as species, tissue type, or the
individual for human cells, if relevant; even primary cultures can become cross-contaminated.
![]() Continuous cell lines should be shown to be authentic using genetic profiling
or an equivalent test of similar stringency.
Continuous cell lines should be shown to be authentic using genetic profiling
or an equivalent test of similar stringency.
![]() Obtaining cell lines from the originator should not be construed as sufficient
evidence of their identity.
Obtaining cell lines from the originator should not be construed as sufficient
evidence of their identity.
![]() Whenever possible, cell lines should be obtained from a major repository that
has a rigorous program of cell-line authentication. Doing so does not absolve
the investigator, however, of the responsibility to confirm independently and
monitor authenticity as experimental use of the cells continues.
Whenever possible, cell lines should be obtained from a major repository that
has a rigorous program of cell-line authentication. Doing so does not absolve
the investigator, however, of the responsibility to confirm independently and
monitor authenticity as experimental use of the cells continues.
Nardone lists various techniques for authentication, such as karyotyping, iso-enzyme profiling (developed by Stephen O’Brien of NCI), and DNA fingerprinting, in his white paper entitled "Eradication of Cross-Contaminated Cell Lines: A Call for Action," now published in Cell Biology and Toxicology and posted here.
DNA profiling, although usually not possible in most small labs, is becoming increasingly affordable to outsource, according to papers by Charles Patrick Reynolds of the University of Southern California in Los Angeles and by John Masters of University College London.
Nardone
and his colleagues recommend short-tandem repeats as an effective method
of profiling. They also recommend improved laboratory practices, such as working
with only one cell line or cell lineage in the hood at one time and never sharing
reagents, what he called the cardinal rule.
![]()