
| T H E N I H C A T A L Y S T | M A R C H – A P R I L 2004 |
|
|
|
| P E O P L E |
RECENTLY
TENURED
 |
|
Tamas
Balla
|
Tamas Balla received his M.D. from Semmelweis University School of Medicine, Budapest, Hungary, in 1979. He earned his Ph.D. from the Hungarian National Academy of Science in 1987. He did his postdoctoral training at NICHD between 1985 and 1987 and returned to NIH in 1989. He became a tenure-track investigator in 1997 in the NICHD Endocrinology and Reproduction Research Branch, where he is currently leading the Molecular Signal Transduction Section.
My engagement with research started when I was invited to join the laboratory of Andras Spat at the Department of Physiology, Semmelweis University, in Budapest as a second-year medical student. At the time, the group was investigating how the renin-angiotensin system regulates secretion of the adrenal mineralocorticoid hormone aldosterone in various forms of sodium deprivation. The importance of this question is underlined by the fact that many of the currently used antihypertensive drugs target the renin-angiotensin-aldosterone axis.
After finishing medical school, I joined the faculty in the physiology department and worked on the mechanism(s) by which the pressor peptide hormone angiotensin II (AngII) increases adrenal steroid secretion. More specifically, I tried to identify the molecular events initiated by the binding of the peptide to its cell-surface receptors.
At that time, little was known about the second messengers that mediate the effects of AngII in its target cells. However, the notion that increased phosphoinositide turnover is an early signaling event in the stimulatory actions of certain hormones and growth factors that elevate cytoplasmic Ca2+ concentration was beginning to emerge. In a series of studies, my colleagues and I established that AngII receptors used the phosphoinositide-Ca2+ signaling cascade in the adrenal, a result that was consistent with other labs’ findings on AngII receptors in other tissues.
 |
When I came to the NIH in 1985 as a postdoctoral fellow in Kevin Catt’s group at the Endocrinology and Reproduction Research Branch of NICHD, I continued to explore the increasing complexity of inositol lipid and phosphate metabolism and its connection to Ca2+ signaling, using the AngII receptors as a model system. Using HPLC to separate the various forms of inositol phosphates in metabolically labeled cells, we clarified the metabolic fate of the second messenger Ins(1,4,5)P3, and we identified additional, highly phosphorylated inositol phosphates in the cells and described a novel pathway connecting them to Ins(1,4,5)P3.
This period was the golden era of research on inositol phosphate–Ca2+ signaling. During this time, scientists described, isolated, and cloned the various forms of the phospholipase C enzymes, the GTP-binding proteins linking the receptors to phospholipase C activation, the protein kinase C isoforms that are activated by diacylglycerol, and the inositol 1,4,5-trisphosphate receptor calcium channels. The excitement and publicity surrounding this research topic was tremendously inspiring and has fueled my engagement in this rapidly expanding research field ever since.
The current work of my research group was initiated by studies in the mid-1990s when we observed that the phosphoinositide precursors for receptor-mediated production of Ins(1,4,5)P3 are synthesized by a phosphatidylinositol 4-kinase (PI4K) activity that is sensitive to PI 3-kinase inhibitors, such as wortmannin. This distinguished it from the other, membrane-bound PI4Ks known at the time. This led our lab to the isolation and molecular cloning of two soluble bovine PI4Ks.
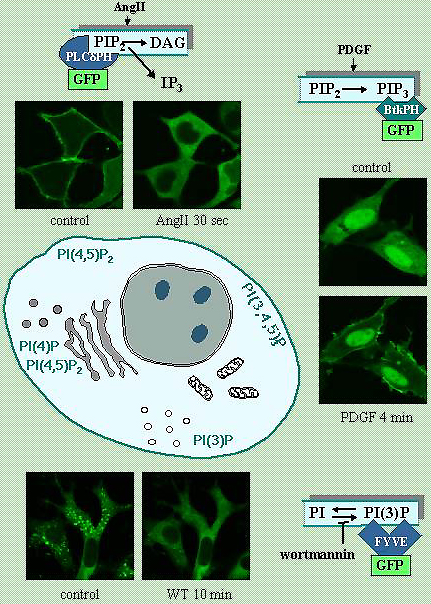
While studying the functions and regulation of these enzymes, which are found in distinct cellular compartments, I explored new ways to examine localized production of inositol lipids and follow their dynamics in intact single cells. We created fusion proteins consisting of the green fluorescent protein and small protein modules known to confer specific inositol lipid interactions. By expressing these modules specific for particular inositol lipids, we were able to track production and localization of several lipids in intact cells (see figure). This technique, which was also developed and used in several other labs, has become a driving force in understanding the spatial and temporal aspects of inositol lipid signals.
Research of the last 10 years has shown that the versatility of inositide-based signaling is almost unparalleled in biology; only the GTP-binding proteins are similar in their ubiquitously important regulatory roles. Inositides are involved in:
![]() Mediating the metabolic, proliferative, and antiapoptotic effects of hormones
Mediating the metabolic, proliferative, and antiapoptotic effects of hormones
![]() Regulating the trafficking of molecules between various organelles
Regulating the trafficking of molecules between various organelles
![]() Controlling exo- and endocytosis
Controlling exo- and endocytosis
![]() Regulating almost all ion channels and transporters
Regulating almost all ion channels and transporters
Inositides have also been implicated in nuclear signaling events. Because of their pleiotropic functions, inositide-regulated processes are often relevant to human diseases, such as diabetes, malignancy, and immunodeficiency. They are also targeted and used by pathogenic agents (bacteria, viruses, and toxins) to gain access to cells, to move around in them, or to be assembled and released from them.
Given such complexity, it’s important to focus on certain areas. Our current research deals with the PI4Ks and their roles in trafficking of G-protein–coupled receptors and with the regulation of the phosphoinositide pools utilized to generate Ca2+ signals. Ongoing studies are aimed at identifying regulatory partners of PI4K enzymes in various cellular compartments. We also are improving and using imaging tools to understand the spatial organization of early signaling events initiated by hormones and neurotransmitters..
 |
|
Amir
Gandjbakhche
|
Amir Gandjbakhche received his Ph.D. from the University of Paris Denis Diderot in 1989. He joined NIH as a postdoctoral fellow in 1990, entered the tenure track in 1999 in the Laboratory of Integrative and Medical Biophysics, NICHD, and is now a senior investigator and chief of the Section on Biomedical Stochastic Physics.
The objectives of the section are to devise theories, develop methodologies, and design instrumentation to study biological phenomena that have elements of randomness in both time and space.
Currently, the main focus of my section is to develop quantitative theories applicable to in vivo quantitative tissue optical spectroscopy and tomographic imaging of tissues. To achieve this goal, we are taking a multifaceted theoretical, computational, experimental, and clinical research approach to bring the methodology and associated technology from bench to bedside.
My key scientific challenge is to use the spectroscopic power of light for in vivo functional imaging of thick tissue and thereby relate metabolic activities at the molecular and cellular level to tissue function and metabolism.
This work requires analysis of different optical sources of contrast, such as absorption (for example, by hemoglobin or varying chromophore concentration) and/or scattering. We emphasize exogenous fluorescent labels as specific contrast agents.
Some current projects and collaborations we’re conducting include:
I. Time-resolved tomography of thick tissue: application to quantitative spectroscopy of breast tumors and the use of specific fluorescent markers for identifying disease processes: This approach uses a transillumination method in which a very short pulse of laser light (of ~picosecond duration) impinges on the tissue. Because of the scattering properties of tissue, photons experience random walks, resulting in temporal dispersion of their time of arrival at the detector, which records light intensities at each time window. This is called time-of-flight (TOF) of photons.
Although direct imaging of abnormalities with high resolution is not possible, this temporal discrimination of photon paths by quantitative spectroscopy allows theoretical constructs that separate the effects of scattering from absorption, in principle yielding optical coefficients that are spectroscopic signatures of abnormal tissue embedded in thick, normal tissue.
I have devised theoretical constructs, based on a random walk theory on a lattice that uses time-dependent contrast functions, to derive optical properties and the size of an abnormal target from TOF data. I have applied this method to quantify optical properties of breast tumors for three patients presenting with invasive ductal carcinoma. The tumors showed increased absorption and scattering. From the absorption coefficients at different wavelengths, we were able to estimate blood oxygen saturation for the tumors and surrounding tissue.
We found that the tumors are hypoxic and their blood volume is increased by about a factor of two compared with surrounding tissue, indicating increased vascularization. I plan to use this technique for monitoring breast cancer patients undergoing chemotherapy and to study metabolic activity in the breast and correlate the results with treatment outcome.
II. 3-D reconstruction of localized fluorescence: The development of fluorescently labeled cell surface markers has opened the possibility of specific, quantitative, and noninvasive diagnosis of tissue changes, the ultimate goal being noninvasive optical biopsy.
Toward this end, I have derived an exact mathematical expression for signals emitted through an optically turbid medium by fluorescent masses. This expression incorporates the dependence of the signal on many parameters, including absorption and scattering coefficients at excitation and emission wavelengths, depth, and quantum yield.
We have begun to apply these findings in a series of animal experiments in which we study the immune response to the presence of squamous cell carcinoma in the tongues of BALB-c mice. We inject the mice with antibodies to CD3 or CD19 conjugated with fluorescent molecules.
I was able to show that my model of diffuse fluorescent photon migration can separate the effects of light diffusion at a given depth from the actual distributions of the fluorescent antibodies, thereby permitting us to measure noninvasively the degree and pattern of antibody binding—essentially permitting us to image the neoplastic cells and the associated pharmacokinetics.
In related work with ORS-DBEPS and NCI collaborators, I am heavily involved in applying these quantitative approaches in the use of new optical molecular contrast agents such as quantum dots. This method has been successfully tested in vivo to study vascularization deep in the thoracic cavities of mice. (See DDIR’s Web Board, January 2004).
III. Monitoring blood circulation in Kaposi’s sarcoma (KS): In collaboration with two NCI clinical protocols, we are evaluating the effectiveness of antiangiogenesis drug treatment of patients with KS.
New drugs targeting angiogenesis or blood flow have led to a need to monitor blood circulation noninvasively. I am investigating the use of three imaging modalities to quantify different parameters associated with blood circulation: 1) thermography, 2) laser Doppler imaging (LDI), which produces two-dimensional images of blood flow over a defined area at 690 nm and 780 nm, and 3) multispectral imaging, which produces two-dimensional reflectance images at six wavelengths (700–1,000 nm, in increments of 50 nm).
I am testing these techniques in an NCI-sponsored clinical trial of antiangiogenic drugs for KS, a highly vascularized tumor in which angiogenesis and capillary permeability can play key roles in disease development and progression. No standard noninvasive technique has yet emerged for the assessment of the effect of antiangiogenic therapy on blood flow to the tumors.
To test the potential applicability of the three noninvasive methods for assessing vascular changes associated with KS, we conduct thermographic, LDI, and multispectral imaging of KS lesions, comparing them with contralateral, lesion-free sites prior to antiangiogenesis chemotherapy and after treatment.
Six patients have now finished the first phase of the treatment, and preliminary results show that lesions with higher temperature and blood flow in the lesion (compared with the contralateral side) are improved after treatment. Confirmation with conventional biopsy suggests that cooler lesions are not responding to the treatment.
Another area of research is devising polarimetric methods to study the destruction of collagen network after radiation treatment.
As demonstrated by this diverse array of collaborative projects, I would conclude that, for investigators who are interested in quantitative, functional, and noninvasive optical methods, this is a great time to collaborate with my section!
 |
|
Sam
Hwang
|
Sam Hwang received his Ph.D. in Biochemistry from the University of Basel (Switzerland) in 1989 and his M.D. from Harvard Medical School in 1991. Following internship at the Brigham and Women’s Hospital in Boston, he trained in dermatology at the University of California, San Francisco. After residency, he was awarded a Howard Hughes Physician Fellowship in the laboratory of Steven Rosen (UCSF). In 1997, he became a tenure-track investigator in the Dermatology Branch of the CCR, NCI, where he is currently a senior investigator.
My laboratory is interested in the roles of chemokine receptors in the biology of normal immune cells and cancer cells. The chemokine receptors form a large family of G-protein–coupled membrane proteins with seven transmembrane-spanning domains. These proteins bind to chemotactic cytokines (chemokines) and are best known for regulating directional migration in response to inflammatory stimuli.
My initial work at NIH addressed the mechanisms by which antigen-presenting cells migrate out of skin under inflammatory conditions and move to secondary lymphoid organs via afferent lymphatic vessels. We found that a specific chemokine receptor, CCR7, and its ligand, CCL21, played critical roles in the directed migration of antigen-presenting dendritic cells from the skin to dermal lymphatic vessels and then to lymph nodes. This was due to the high expression of CCL21 by lymphatic endothelial cells and nodal stromal cells.
Based on prior work in leukocytes demonstrating the important roles of chemokine receptors in organ-selective leukocyte trafficking, we hypothesized that specific expression of certain chemokine receptors may facilitate cancer metastasis. In support of this supposition, preliminary studies in our lab indicated that human melanoma cell lines expressed CCR7 as well as the receptors CXCR4 and CCR10.
Interestingly, others have shown that the ligand for each of these receptors is expressed at high levels at common sites of melanoma metastasis. For example, CXCR4 ligand (CXCL12) is found in the lung, and CCR10 ligand (CCL27) is constitutively expressed in the skin.
To determine the function of these receptors in cancer cells, we overex-pressed them in B16 murine melanoma cells (where they are usually absent) and then assessed changes in the trafficking or survival pathways of the transfected cells. Compared with control cells, B16 cells overexpressing CCR7 (CCR7-B16 cells) metastasized readily to the skin-draining lymph node after cutaneous implantation. In contrast, compared with control cells, CXCR4-B16 cells showed 6- to 10-fold increases in pulmonary metastasis and adhered far more efficiently to pulmonary endothelial cells in vitro.
To make these findings, we took advantage of our experience with an advanced in vitro imaging system that allowed us to observe and quantify real-time interactions of tumor cells with endothelial cells under shear stress conditions. This system allowed more realistic modeling of the interactions of tumor cells with vascular endothelial cells that occur in complex vascular spaces in vivo.
Skin is a common site for the metastasis of melanoma. We suspected that CCR10 played a role in skin metastasis because keratinocytes in skin constitutively produce the CCR10 ligand, CCL27. In vivo, CCR10-B16 cells formed progressive tumors in the skin, whereas control tumor cells could not. Skin-derived CCL27 contributed to tumor formation of CCR10-B16 cells, because neutralizing anti-CCL27 antibodies completely blocked tumor formation in the cutaneous environment.
In these experiments, we also determined that CCR10 activation led to striking resistance of tumor cells to apoptosis mediated by Fas-ligation and by exposure to cytotoxic CD8 T cells that target cells bearing melanocyte antigens. The resistance to cell killing was likely due to an observed increase in the activation of Akt (a known antiapoptotic signaling molecule) in B16 cells after CCR10 activation.
Taken together, our results suggest that chemokine receptors may effectively help cancer cells evade important immune cell killing mechanisms.
Our search for mechanisms by which immune cells move into and out of the skin has resulted in a new set of questions pertaining to the molecular basis for organ-selective metastasis. We have discovered that normal and malignant cells, to a striking degree, share certain trafficking mechanisms.
The most clinically promising result from this work is our demonstration that chemokine receptor activation can render cancer cells more resistant to apoptotic signals.
Indeed, my laboratory
is now seeking to prove a corollary of that result, namely, that chemokine receptor
inhibitors will increase the efficacy of conventional apoptosis-inducing therapies,
such as chemotherapy or radiation in clinical treatment of cancer patients.
![]()