
| T H E N I H C A T A L Y S T | S E P T E M B E R – O C T O B E R 1999 |
|
|
|
|
by Tory Hampshire,
DVM, NINDS |
Hot
Mouse Tips: a Three-Part Series
PART
2. THE INS AND OUTS
OF SUCCESSFUL SURGERY
 |
In the first article of this series (NIH Catalyst, May–June 1999), we provided an overview of normal physiological responses of mice and rats and expected deviations from normal after invasive procedures. We also made suggestions regarding preanesthetic preparation, anesthetic induction, and anesthetic maintenance. This article discusses in greater detail components of successful rodent surgery.http://www.nih.gov/catalyst/1999/99.05.01/page5.html
Performing successful surgical procedures on rodents to obtain valid and relevant experimental data, depends on not only the necessary technical equipment but also surgeons with motivation, a certain persistence, and a genuine interest. Successful surgeries may also require training, qualified supervision, and a positive attitude to teamwork.
The investigator willing to learn to use state-of-the-art inhalation-anesthesia equipment will enjoy easily controlled anesthesia with remarkably fast induction and wake-up times.
For details beyond the "helpful hints" of this article, see the handout Rodent Anesthesia for the Investigator, available from the NINDS Animal Health Care Section.
 |
|
Magnification
glasses afford the user more freedom of movement and good field of view.
These 4.5x glasses are by Designs for Visions.
|
Seeing Is Believing
Tiny blood vessels and organs require good visualization and comfortable work surfaces to reduce frustration for the scientist. Key technical requirements include:
![]() Microscopes. Operating microscopes or magnifying glasses enhance vision
and freedom of movement.
Microscopes. Operating microscopes or magnifying glasses enhance vision
and freedom of movement.
![]() Microsurgical instruments and accessories. A few high-quality instruments
are usually sufficient; as a rule, the simpler the better. The choice of optimal
clamps, retractors, catheters, and suture material and needles must also be
considered.
Microsurgical instruments and accessories. A few high-quality instruments
are usually sufficient; as a rule, the simpler the better. The choice of optimal
clamps, retractors, catheters, and suture material and needles must also be
considered.
![]() A ventilator for long anesthetic procedures.
A ventilator for long anesthetic procedures.
![]() A gas isoflurane anesthesia machine.
A gas isoflurane anesthesia machine.
![]() Small-gauge catheters.
Small-gauge catheters.
![]() Heparin solutions.
Heparin solutions.
![]() Banked blood from like genotypes.
Banked blood from like genotypes.
Basic Techniques and Considerations
The considerably higher success rate in human anesthesia compared to animal anesthesia seems to underline the inherent safety of properly administered anesthesia and highlights the value of intense monitoring during recovery that occurs with humans. In rodent surgery, many scientists are often guilty of poor selection of anesthetic agents and insufficient physiological monitoring and surgical techniques. Helpful Hints include:
![]() Better Base-line Evaluations. How does your mouse really look
compared with a normal mouse? If you don’t know what normal is, take the
mouse out of the cage. Compare it with a wild-type mouse by assessing behavior,
gait, and posture. Try to think of all the physiologic and anatomic outcomes
your genetic alteration might cause. Talk to a clinician in that field and determine
a test battery that might be used to explore that mutation in a human. Then
discuss with your institute vet which tests could be done in mice. Details might
surprise you. Physical conditions of genetically altered mice may significantly
alter anesthesia regimens. For example, we are just beginning to get electromyographic
and nerve studies on neurologically altered mice in our institute. The effect
of peripheral neuropathies on respiratory function is a concern for anesthetic
studies. Muscle weakness associated with these conditions reduces the functional
residual capacity and depresses alveolar ventilation. Other contributing factors
to consider would be health status, age, and animal position for surgery. For
example, pregnant animals frequently do not have adequate tidal volume due to
pressure on the diaphragm. Older animals with less compliant lungs may have
inadequate gas exchange despite a relatively normal tidal volume and respiratory
rate. Geriatric animals (a 1.5- to 2-year–old stock mouse or rat is old!)
that are anesthetized and breathing room air are more prone to hypoxemia than
young animals. Note that age ranges vary with strain; R111 mice, for example,
typically live no longer than 8 to 10 months.
Better Base-line Evaluations. How does your mouse really look
compared with a normal mouse? If you don’t know what normal is, take the
mouse out of the cage. Compare it with a wild-type mouse by assessing behavior,
gait, and posture. Try to think of all the physiologic and anatomic outcomes
your genetic alteration might cause. Talk to a clinician in that field and determine
a test battery that might be used to explore that mutation in a human. Then
discuss with your institute vet which tests could be done in mice. Details might
surprise you. Physical conditions of genetically altered mice may significantly
alter anesthesia regimens. For example, we are just beginning to get electromyographic
and nerve studies on neurologically altered mice in our institute. The effect
of peripheral neuropathies on respiratory function is a concern for anesthetic
studies. Muscle weakness associated with these conditions reduces the functional
residual capacity and depresses alveolar ventilation. Other contributing factors
to consider would be health status, age, and animal position for surgery. For
example, pregnant animals frequently do not have adequate tidal volume due to
pressure on the diaphragm. Older animals with less compliant lungs may have
inadequate gas exchange despite a relatively normal tidal volume and respiratory
rate. Geriatric animals (a 1.5- to 2-year–old stock mouse or rat is old!)
that are anesthetized and breathing room air are more prone to hypoxemia than
young animals. Note that age ranges vary with strain; R111 mice, for example,
typically live no longer than 8 to 10 months.
![]() Chronic or Acute: Is the Mouse Here to Stay?
Most discussions of anesthesia in animals fail to distinguish the marked
differences between the anesthetic technique for acute (nonrecovery) and chronic
(recovery) experiments. The two experimental conditions require different techniques
and anesthetic considerations. For example, interpretation of data derived from
acute experiments using barbiturate or a-chloralose type anesthesia is difficult
because of the animal’s constantly changing homeostatic background. The
popularity of a-chloralose for cardiovascular and neurophysiological studies
is based on the drug’s apparent lack of reflex and cardiovascular depression,
an advantage over barbiturates. However, when attempts are made to keep a constant
level of anesthesia by infusion over a three-hour period, there can be marked
fluctuations in cardiovascular parameters.
Chronic or Acute: Is the Mouse Here to Stay?
Most discussions of anesthesia in animals fail to distinguish the marked
differences between the anesthetic technique for acute (nonrecovery) and chronic
(recovery) experiments. The two experimental conditions require different techniques
and anesthetic considerations. For example, interpretation of data derived from
acute experiments using barbiturate or a-chloralose type anesthesia is difficult
because of the animal’s constantly changing homeostatic background. The
popularity of a-chloralose for cardiovascular and neurophysiological studies
is based on the drug’s apparent lack of reflex and cardiovascular depression,
an advantage over barbiturates. However, when attempts are made to keep a constant
level of anesthesia by infusion over a three-hour period, there can be marked
fluctuations in cardiovascular parameters.
![]() Gentle Handling. Atraumatic technique is an essential prerequisite
for successful microsurgical procedures. For example, blunt dissection of arteries
and veins and unnecessary manipulation must be avoided. The choice of clamps
must be adapted to the type and size of the vessel; an optimal clamp exerts
a minimal degree of compression, diminishing the risk of endothelial damage.
Any instrumental manipulation with risk of intimal damage during preparation,
as well as suturing, increases the possibility for thrombosis. When scheduling
surgery, allow plenty of time to handle tissue gently.
Gentle Handling. Atraumatic technique is an essential prerequisite
for successful microsurgical procedures. For example, blunt dissection of arteries
and veins and unnecessary manipulation must be avoided. The choice of clamps
must be adapted to the type and size of the vessel; an optimal clamp exerts
a minimal degree of compression, diminishing the risk of endothelial damage.
Any instrumental manipulation with risk of intimal damage during preparation,
as well as suturing, increases the possibility for thrombosis. When scheduling
surgery, allow plenty of time to handle tissue gently.
Machines and Agents
 |
|
A
simple isoflurane induction chamber, oxygen flow meter, and kettle beside
the flow meter
|
As we discussed in our first article, the most consistent and reliable anesthetic protocols for rodents involve inhalation anesthesia for both induction and maintenance, coupled with vigilant monitoring. Isoflurane with a calibrated vaporizer is the method of choice in terms of patient safety and lack of environmental contamination. We are not recommending the open-drop method (using ether, methoxyflurane, or halothane in bell jars), with its associated risks stemming from anesthetic flammability, waste-gas scavenging problems (including toxicity to humans handling methoxyflurane), and unacceptable mortality rates. As with injectables, open-drop methods are also associated with fluctuating anesthetic depth.
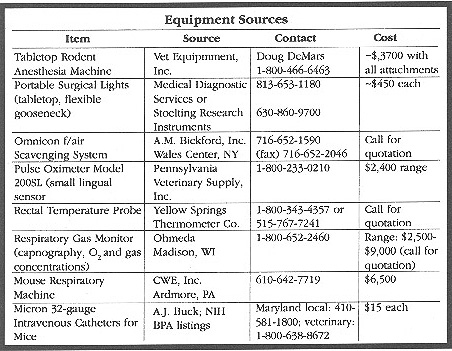
![]() Purchasing the Machine. You will need a
standard isoflurane kettle with flow meter. Oxygen tanks and tank regulators
of the "E-type" can be purchased from Robert’s oxygen. Other
key procurement details are noted at the end of this article. Helpful Hint:
Purchase a portable Rubbermaid cart to secure the oxygen tank, anesthesia machine,
and tubing, as well as other monitoring
equipment, so that you can move between laboratories if necessary. If scavenging
isn’t available at all your potential laboratory sites, purchase charcoal-scavenging
cannisters from almost any veterinary source through blanket purchase agreement
and follow the standard operating procedures for disposal when they reach weight
capacity. The NIH Office of Research Services, Division
of Public Safety will send a contract-monitoring representative to check
the safety of this kind of system.
Purchasing the Machine. You will need a
standard isoflurane kettle with flow meter. Oxygen tanks and tank regulators
of the "E-type" can be purchased from Robert’s oxygen. Other
key procurement details are noted at the end of this article. Helpful Hint:
Purchase a portable Rubbermaid cart to secure the oxygen tank, anesthesia machine,
and tubing, as well as other monitoring
equipment, so that you can move between laboratories if necessary. If scavenging
isn’t available at all your potential laboratory sites, purchase charcoal-scavenging
cannisters from almost any veterinary source through blanket purchase agreement
and follow the standard operating procedures for disposal when they reach weight
capacity. The NIH Office of Research Services, Division
of Public Safety will send a contract-monitoring representative to check
the safety of this kind of system.
 |
||
|
This
handmade face mask has two lines: one line
from the inlet gas . . . |
.
. .and the second to the facility exhaust.
|
A
Plexiglas induction box inlet can be attached to the face mask when the
mouse is alseep
|
|
You
can even make a face mask with PVC or Plexiglas tubing. Take the mouse
out of the induction chamber and connect the outflow directly to a scavenger
system and the inflow port to the common gas outlet, using a "Y"
connector and tubing. The tubing should have a stop-valve control for
delivery to either the induction chamber or the face mask (or nonrebreathing
tube). Induction chambers should allow continuous observation of the animal.
Watch the animal!
|
||
![]() Induction
Box. Inhalation anesthesia can be delivered directly to the
animal through use of a small Plexiglas box (induction chamber) with an inlet
and outlet port. The chamber should be the appropriate size for the animal!
Induction
Box. Inhalation anesthesia can be delivered directly to the
animal through use of a small Plexiglas box (induction chamber) with an inlet
and outlet port. The chamber should be the appropriate size for the animal!
![]() Mechanical Ventilation: Endotracheal Tube Intubation.
Delivery of nhalation anesthesia can also be accomplished by using an endotracheal
tube (ETT). Although this technique requires considerable practice, proper equipment
and experience lead to successful, quick intubation in many animals. For projects
requiring a ventilator or long-term anesthesia, an ETT may be your best choice.
There is no ideal ETT. For rats, a 14-gauge or 16-gauge IV catheter can be used,
as can a modified Cook Flexi-Tip"
Urethral Catheter (V-021305). The advantages are that they require no stylet,
are reusable, and are less traumatic. The disadvantage is that they are more
expensive than IV catheters. You can also use silastic tubing (for a mouse,
0.025" i.d., 0.047" o.d., and for a rat, 0.040" i.d., 0.085"
o.d.). IV catheters (20 or 22 gauge) will also work for mice. Measure the distance
from the mouth to the cricoid cartilage (20 mm for a 25- to 35-g mouse; 26 mm
for a 250- to 300-g rat).
Mechanical Ventilation: Endotracheal Tube Intubation.
Delivery of nhalation anesthesia can also be accomplished by using an endotracheal
tube (ETT). Although this technique requires considerable practice, proper equipment
and experience lead to successful, quick intubation in many animals. For projects
requiring a ventilator or long-term anesthesia, an ETT may be your best choice.
There is no ideal ETT. For rats, a 14-gauge or 16-gauge IV catheter can be used,
as can a modified Cook Flexi-Tip"
Urethral Catheter (V-021305). The advantages are that they require no stylet,
are reusable, and are less traumatic. The disadvantage is that they are more
expensive than IV catheters. You can also use silastic tubing (for a mouse,
0.025" i.d., 0.047" o.d., and for a rat, 0.040" i.d., 0.085"
o.d.). IV catheters (20 or 22 gauge) will also work for mice. Measure the distance
from the mouth to the cricoid cartilage (20 mm for a 25- to 35-g mouse; 26 mm
for a 250- to 300-g rat).
 |
|
Visualization
of the trachea and insertion of the endotracheal tube: animal is supine,
its jaws held open with rubber bands and cheek folds spread with a canary
beak spreader—or by an assistant standing behind you.
|
 |
|
Multiple
mice can be anesthetized simultaneously using a handmade manifold from
the standard kettle and flowmeter over a downdraft surface.
|
Mechanical ventilation can be achieved by invasive or noninvasive means. Invasive mechanical ventilation requires deep induction with isoflurane or injectable components and rapid cannulation of the trachea using a 20-gauge IV catheter sleeve as an endotracheal tube. Again, this technique takes a lot of practice in mice and requires magnification. Risks include trauma to the tongue, cheek pouches, and pharynx and may become problematic. Catheters can be sealed by premeasuring from the bottom of the hub to the thoracic inlet and placing a thin bead of silicone around the catheter. Allow it to dry 24 hours before using.
|
Noninvasive
mechanical ventilation is very attractive for chronic recovery models
in the mouse. The procedure can be achieved atraumatically by setting
the ventilatory rate around 120/min and the volume at 10 mL/kg and using
a tightly sealed face mask. The mask must be custom-made and uses the
mouse’s loose skin as an "auto-seal." For the average 30-g
adult mouse, a 6-mL syringe case works well.
|
Move the tongue to the side with a dry cotton-tipped applicator, with the animal resting on its breastbone. Insert an otoscope while tilting the animal’s head to a 45° angle. Avoid traumatizing the soft palate but place the otoscope at the base of the tongue. Then elevate the head to an 80° angle, forcing the epiglottis to protrude away from the mucous membranes and allowing complete visualization of the laryngeal folds. Carefully advance the ETT to the laryngeal folds and then remove the otoscope. Quickly advance the ETT past the laryngeal folds. If you feel some resistance, rotate the tube as you advance it. You will feel a "pop" as you enter the trachea. Check the tube for patency (wisp of cotton held at the end of the tube). Secure the ETT with tape or suture it to the lip.
Proper positioning of the rodent and good magnification are key elements in successful intubation; 4X magnification glasses are perfect for this feat and give the scientist more freedom of movement (they can be purchased from Designs for Visions—516-585-3404). Alternatively, an operating scope with a horizontal arm 4X beam will also work. Both require practice. In the beginning, it is best to use injectable anesthetics to hold the mouse in a deep enough plane of anesthesia for training. Invasive me chanical ventilation by endotracheal intubation is best done in acute mouse models. Access may be gained by tracheotomy—a surgical incision into the trachea through which an ETT is inserted.
|
Intravascular
cannulation for measurement of blood gases can also be achieved with lots
of practice. New catheters have been designed by Micron (available through
A.J.Buck on BPA at NIH) that are 32 gauge and can be fed through a 26-gauge
introducer needle in the internal carotid or jugular. With great practice,
the femoral vein or artery can also be cannulated. Because of the small
size of the vessels, it is imperative that a flush be at hand, and that
the mouse is heparinized before manipulation (400 mg/kg
subcutaneously five minutes before cannulation will work). If you plan
on performing long procedures and sampling, you should have like-strain
blood replacement available. Because of the small amount of blood volume
in the mouse (1.0 mL) and the relatively large size of the sample for
blood gas measurement (0.1–0.2 mL), combined with the dead space
in the lines, the risk of hypovolemic shock warrants ready access to blood
replacement.
|
![]() Vaporizers. Vaporizers facilitate
evaporation of an anesthetic liquid and control the concentration of anesthetic
in the carrier gas. Vaporization of volatile anesthetics depends on vapor pressure
and ambient temperature. Vaporizers are calibrated for the agent for which they
were designed because anesthetic agents differ in their vapor pressure and heat
of vaporization. If you fill a vaporizer that was designed to deliver one agent,
for instance, methoxyflurane, to deliver another agent, isoflurane, you cannot
rely upon the concentration you set because the vapor pressure of methoxyflurane
is 23 mmHg at 20° C, while that for isoflurane is
240 mmHg at 20° C. In contrast, the vapor pressure
of isoflurane and halothane are similar; therefore, isoflurane can be accurately
delivered from a vaporizer calibrated for halothane. However, companies (such
as Anesthetic Vaporizer Services) will do changeovers for a reasonable cost.
Vaporizers. Vaporizers facilitate
evaporation of an anesthetic liquid and control the concentration of anesthetic
in the carrier gas. Vaporization of volatile anesthetics depends on vapor pressure
and ambient temperature. Vaporizers are calibrated for the agent for which they
were designed because anesthetic agents differ in their vapor pressure and heat
of vaporization. If you fill a vaporizer that was designed to deliver one agent,
for instance, methoxyflurane, to deliver another agent, isoflurane, you cannot
rely upon the concentration you set because the vapor pressure of methoxyflurane
is 23 mmHg at 20° C, while that for isoflurane is
240 mmHg at 20° C. In contrast, the vapor pressure
of isoflurane and halothane are similar; therefore, isoflurane can be accurately
delivered from a vaporizer calibrated for halothane. However, companies (such
as Anesthetic Vaporizer Services) will do changeovers for a reasonable cost.
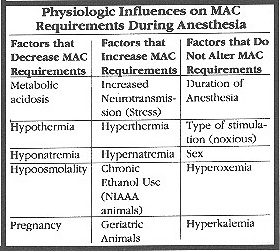
As a rule, a vaporizer setting of 1 X minimum alveolar concentration (MAC) will produce light anesthesia. 1.5 X MAC will produce a surgical depth of anesthesia, and 2 X MAC will produce deep anesthesia. Inhalant potency is fairly constant across species, so vaporizer settings for inhalants will be fairly consistent with those of more familiar species.
![]() Rates and Volumes. Commercial ventilators,
like the CWE distributed through Harvard Biosciences, usually have either or
both pressure and volume deliveries. Pressure-driven ventilators trigger a burst
for inhalation that is based on negative draw of the chest wall; they require
a tight seal of the ETT and no use of paralytic agents. These are advantages
for short procedures because a balanced mouse patient will trigger the machine
at will. Remember, if the rate slows drastically during anesthesia, the mouse
will not compensate with increased tidal volume and is prone to develop hypoxia.
It may also inspire less anesthetic gas and become less anesthetized. A pressure-driven
ventilator also may cause less alveolar damage. Volume ventilation settings,
on the other hand, deliver set volumes at established rates. They are ideal
for long procedures and will work with imperfect seals. Many newer models have
both options. Weigh the mouse to achieve the most reasonable tidal volume (10
mL X body weight in kg). Tidal volume is discussed in detail in our handout.
Such methods as counting the respiratory rate and evaluating the color of mucous
membranes are commonly used but are not practical in rodents.
Rates and Volumes. Commercial ventilators,
like the CWE distributed through Harvard Biosciences, usually have either or
both pressure and volume deliveries. Pressure-driven ventilators trigger a burst
for inhalation that is based on negative draw of the chest wall; they require
a tight seal of the ETT and no use of paralytic agents. These are advantages
for short procedures because a balanced mouse patient will trigger the machine
at will. Remember, if the rate slows drastically during anesthesia, the mouse
will not compensate with increased tidal volume and is prone to develop hypoxia.
It may also inspire less anesthetic gas and become less anesthetized. A pressure-driven
ventilator also may cause less alveolar damage. Volume ventilation settings,
on the other hand, deliver set volumes at established rates. They are ideal
for long procedures and will work with imperfect seals. Many newer models have
both options. Weigh the mouse to achieve the most reasonable tidal volume (10
mL X body weight in kg). Tidal volume is discussed in detail in our handout.
Such methods as counting the respiratory rate and evaluating the color of mucous
membranes are commonly used but are not practical in rodents. ![]()
Disclaimer: Mention of specific products in this article does not constitute an endorsement of those products, nor does it signify that other similar products are less desirable.
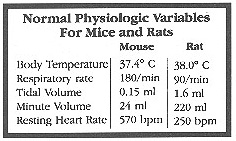
From Flecknell, P.A. Laboratory Animal Anesthesia.
Academic Press, San Diego, CA. 1992
|
 |
 |