
| T H E N I H C A T A L Y S T | J A N U A R Y – F E B R U A R Y 1999 |
|
|
|
NIH TAKES ON TRANSPLANTATION AS PART OF NIDDK-NAVY PROGRAMTO TACKLE TYPE 1 DIABETES AND OTHER AUTOIMMUNE DISEASES |
by Fran Pollner |
 |
|
Allen
Spiegel
|
The NIH Clinical Center will exit the 20th century as an active organ- and tissue-transplant center, with several dozen novel kidney and pancreatic islet transplantions expected to be carried out there between May and year’s end.
The operations will take place in an NIDDK abuzz with intellectual excitement over immune-modulating therapies that may eliminate the need for immunosuppressive drugs to prevent graft rejection. A renovated Ward 11 East in Building 10 is becoming a dedicated transplant site, a nexus for the translational research that will find its way from the laboratory of a new NIDDK research branch into clinical studies.
Opportunity knocked three times at once to inspire the creation of such a program, according to NIDDK scientific director Allen Spiegel: an overarching renewal of appreciation for clinical research in general and for the need to expand research on the pathogenesis and treatment of type 1 diabetes in particular; results compelling enough in animals to warrant clinical studies of a new approach to kidney and islet cell transplantation; and coincidental plans to reorganize both the NIH Clinical Center and the military medical research establishment that eased the way to a collaborative effort.
The new Transplantation and Autoimmunity Research Branch is a joint NIDDK-Navy venture. It was initiated by Spiegel, who enlisted the Navy personnel and orchestrated the project that would entail securing UNOS (United Network for Organ Sharing) certification and NIDDK collaboration with an array of entities both outside and within NIH.
In the former category are the Navy, the Army, the University of Wisconsin, the University of Miami, and the industry partners of the Navy researchers. In the latter are the Clinical Center, NCI and NHLBI bone marrow transplant researchers, and the NIH stem-cell-processing apparatus. Moreover, if early clinical (phase 1 and 2) trials here on campus produce the kinds of results that all involved parties are betting on, NIAID’s newly formed Collaborative Network for Clinical Research on Immune Tolerance may well be pressed into action to conduct multicenter, phase 3 clinical trials.
 |
|
Dave
Harlan (left) and Allan Kirk
|
The program will "have ripple effects at NIH that go well beyond NIDDK," says Spiegel, whose excitement over the project is tempered only by concern that his own role not be overemphasized. "I fostered this initiative," he says, "but it’s not my science. The science belongs to the principal investigators"—Dave Harlan and Allan Kirk, Navy researchers whose studies of reagents to block the costimulatory pathway in the immune response have resulted in the acceptance without immune suppression of mismatched kidney and pancreatic allografts in rhesus monkeys. "Everything they do—kidney and islet cell transplantation and research into the pathogenesis of type 1 diabetes—could not be more programmatically relevant to what we do," Spiegel notes.
UNOS certification, he adds, was spearheaded by Dave Henderson, CC deputy director for clinical care, who, together with NIDDK chief administrator Barbara Merchant, mobilized the Clinical Center to secure needed space, equipment, and staff. Henderson, in turn, points to the commitment of the CC staff and the "uncompromising support" of CC Director John Gallin as the forces behind the rapid changes. And PIs Harlan and Kirk marvel at the "model of cooperation" exhibited by the civilian and military parties in organizing the project—"all crossing boundaries to achieve the same goals, all driven by enthusiasm for the promise of the science," says Kirk.
That enthusiasm greeted Harlan, Kirk, and Spiegel throughout 1998, as they delivered multiple presentations on the science, logistics, and promise of the bench-to-bedside initiative. They spoke before the Clinical Center Advisory Council, the Medical Board, a Clinical Center town meeting, bench-to-bedside grand rounds, and gatherings of institute and scientific directors. At no point was the science questioned or approval withheld. Rather, there was activity in all quarters to make things happen.
The new Transplantation and Autoimmunity Research Branch will be housed physically across Rockville Pike at the National Naval Medical Center’s Armed Forces Radiobiology Research Institute (AFRRI)–4,000 square feet of laboratory and office space and an adjoining veterinary facility that is being renovated at NIDDK expense and should be available by late fall. "Their retrenchment is an opportunity for us," Spiegel remarks of the military’s string of base closures that includes shutting down the Naval Medical Research Institute and the consolidation of Army and Navy research laboratories at a joint site in Silver Spring, Maryland.
|
"WE'RE NOT TRYING TO BE A LARGE . . . ENTERPRISE, BUT TO TAKE SPECIFIC IDENTIFIED PROBLEMS AND TRANSITION BENCH RESEARCH INTO THE CLINIC FOR THE FIRST TIME. AND WHEN WE FIND THINGS THAT LOOK PROMISING, WE WANT TO DELIVER THEM TO THE EXTRAMURAL COMMUNITY IN AN EXPEDIENT FASHION. . . ." —Allan Kirk |
The preclinical work—in the laboratory and with small and large animals—will unfold at the AFRRI site and then move into the Clinical Center, along with Harlan and Kirk, for testing in humans. "Bench and clinical research go hand-in-hand," Harlan notes, "and we go with it."
Harlan has been nominated to assume the branch chief position and with that change has applied for transfer from the Navy to NIH. Kirk will head the branch transplant section while maintaining his Navy status. "Both the Navy and the Public Health Service recognize that it’s a more logical fit for me to be with the PHS," says Harlan, who heads the Navy’s immune cell biology program and whose research and intense interest in type 1 diabetes gained him a seat several years ago as the Department of Defense liaison on the NIDDK Advisory Council.
His primary objective as an endocrinologist, he says, is to "see a new treatment and potential cure" for type 1 diabetes. While transplantation is clearly a focus of the new branch–and the team expects to perform between 50 and 100 kidney and islet cell transplants a year—"don’t forget that this is a transplantation and autoimmunity branch; we anticipate protocols down the road involving type 1 diabetes markers—and other autoimmune diseases will come along," Harlan says.
Moreover, even protocols involving transplant patients may not necessarily entail transplantation. Subsets of pre- and post-transplant patients present unique and challenging problems, Kirk observes. On the one hand are patients who appear immunologically unfit for transplant and on the other are those vulnerable to chronic rejection after successful transplant. There will be protocols in these areas as well as protocols to introduce novel immune-modulating approaches to kidney and islet cell transplant procedures.
"There are many reasons a transplant center at NIH makes sense," says Kirk, a transplantation surgeon with a commitment to research. "There are a number of therapies we’re working on that look promising for revolutionizing the way transplantation is done, and there are a lot of very good basic investigators in transplant immunology at NIH right now who have no place to go when they want to transition their bench research into the clinic."
Current methods to prevent rejection, he notes, work well against T-cell–mediated rejection but not against antibody-mediated rejection, the problem of about 30 percent of patients on the kidney transplant waiting list. But since this "presensitized" population amounts to no greater than perhaps 10,000 patients, it does not inspire commercial interest. Similarly, the extramural community does not fund "proof-of-concept fishing expeditions" to identify reagents that modulate antibody response. But NIH, Kirk observes, has always been the ideal venue for research that industry and academia cannot easily accommodate. "We’re not trying to be a large, aggressive, commercial enterprise, but to take specific identified problems and transition bench research into the clinic for the first time. And when we find things that look promising, we want to deliver them to the extramural community in an expedient fashion so they can be assayed in large multicentered trials," he says, referring to the NIAID network.
The Science
Costimulatory blockade, the anti-rejection strategy used by Harlan and Kirk, is believed to support long-term graft function through mechanisms other than immune suppression. As the researchers explain it, immune mechanisms are triggered when a specific antigen-presenting cell meets a T-cell receptor on the host T-cell. The costimulatory event, nonspecific but necessary to trigger the immune response, involves one protein on the antigen-presenting cell and one protein on the T-cell. If the first meeting occurs but the costimulatory pathway is blocked, that is "not a neutral event," Spiegel notes. Rather, says Harlan, the "immune response against that specific antigen does not occur, and this immune system inactivity appears to be quite stable." Costimulatory blockade, then, blocks graft rejection not by inducing global immune suppression, as current drugs do, but by creating what the investigators call "immune re-education." Clinical testing of monoclonal antibodies against these costimulatory factors is necessarily complex: target, timing, dose, and duration must all be determined.
The rationale behind costimulatory blockade, Kirk notes, "is to use the physiologic mechanisms of immune modulation to teach the immune system that the organ that has been transplanted is a benign functional organ that should not be removed. Conventional immune suppression "doesn’t take advantage of our immune system’s ability to learn" Kirk notes, "but some fairly fundamental advances in the field in the last 10 years have revealed how the system teaches itself what to respond and not respond to." Harlan points to current and former NIH investigators who have been "leading the way—Ron Schwartz, Mark Jenkins, Al Singer, Richard Hodes, Polly Matzinger, Helen Quill . . . ."
Based on in vitro and mouse studies of other investigators, Harlan and Kirk pioneered costimulatory blockade in nonhuman primates, testing the ability of anti-CD40 ligand to protect against rejection of mismatched kidney allografts in rhesus monkeys. Not only are the animals surviving and maintaining their new organs, they are doing so without any immunosuppressive therapy. Moreover, there have been no signs of infection, complications, or side effects. Such results, Spiegel says, are "compelling," even though the numbers are small. The team had similar success in the islet cell transplant arena in studies done in collaboration with Norma Kenyon and Camillo Ricordi, colleagues at the University of Miami Diabetes Research Institute, who demonstrated that costimulatory blockade after transplanting insulin-producing islets from mismatched donor monkeys into pancreactomized monkeys consistently lead to insulin independence.
Harlan and Kirk began their collaboration several years ago, when Kirk was about to begin a transplantation fellowship under Stuart Knecthle at the University of Wisconsin. Harlan had been doing research on the costimulatory pathway and had been looking for a "gifted academic surgeon when, as an answer beyond my wildest dreams, Dr. Kirk knocked on the door. He was about to start his fellowship, had a four-year Navy obligation, and was looking for a way to work with us—to do both transplantation clinically and be involved in a research program," Harlan recounts.
In concert with commercial CRADA partners, the Navy-Wisconsin team tested several reagents that appeared to have efficacy in preventing rejection of transplanted organs, including various forms of anti-CD40 ligand (renamed anti-CD154) and anti-B7.
Thus far, the longest kidney graft survival in the rhesus monkeys—with no signs of toxicity and no need for immune suppression—is approaching two years; the longest followup for pancreatic islet cells is now one year.
The investigators are well aware, however, of the differences between these healthy test animals whose organs have been removed immediately prior to the transplant procedure and humans with conditions that have destroyed their kidneys or islet cells. "There are arguments for and against the idea that the reagents we’ve been testing will thwart an immune system already activated against the insulin-producing pancreatic b cells, the mechanism underlying type 1 diabetes, Harlan notes, "but everyone agrees it must be tested in humans. There is no primate model for type 1 diabetes."
In creating clinical protocols for costimulatory blockade, there will be one complexity in the kidney transplant setting different from the usual approach to testing new anti-rejection therapies. Typically, a new therapy is offered in addition to conventional immunotherapy to assess whether it improves on the standard treatment. The costimulatory blockade strategy, however, demands that conventional immunosuppression be abandoned because the initial stimulus of antigen meeting T-cell is required for the costimulatory pathway agents to work. Spiegel suggests that other agents more compatible with these mechanisms might be used in the kidney transplant setting.
Logistics
 |
|
Dave
Henderson
|
Some initial kidney transplantation procedures might be performed at the University of Wisconsin and at the Walter Reed Army Medical Center, which is a collaborator in the NIDDK-Navy initiative. Further, preliminary clinical islet transplant studies using costimulatory-pathway-based strategies will likely soon be performed at the University of Miami. For the NIH initiative, Walter Reed is providing its already certified tissue-typing laboratory, which was a prerequisite for UNOS certification, as well as two expert transplant surgeons who, with Kirk, will round out the surgical team initially. The Clinical Center is hiring new staff for inpatient nursing, OR, anesthesia, and other initiative-related positions—as is NIDDK. The OR and anesthesia staff may get their transplantation feet wet at Walter Reed and bring their expertise back to the Clinical Center. The transition, however, should be "seamless," says the CC’s Henderson, who several months ago instituted weekly meetings of a "users group" that includes all CC department heads with a "stake in the program, who might need new or modified resources—pharmacy, critical care medicine, nursing, social work, rehabilitation medicine, anesthesiology, surgical services"—as well as the PIs and NIDDK, CC, and Walter Reed personnel.
There have also been discussions with NCI’s Ron Gress, NHLBI’s John Barrett, and the Diabetes Research Institute’s Ricordi on the role of bone marrow transplant in conjunction with the new transplantation approaches to establish "microchimerism," or an increased tolerance to donor tissue through exposure to the donor’s bone marrow cells. In addition, the Navy’s John Chute is working with the University of Miami investigators to incorporate expanded stem cells in these studies.
Another critical piece of the project is harvesting and transplanting the pancreatic islet cells in a way that keeps them viable up to and during their delivery into the portal vein. Initially, according to Spiegel, islets for the NIDDK-Navy program will be shipped to NIH after harvest at the Miami facility—where the automated islet-isolation technique commonly used in the research setting was developed by Ricordi. But the Clinical Center’s stem-cell-processing facility is "ideal technically and in every other way for islet harvesting," Spiegel notes, "so [transfusion medicine chief] Harvey Klein is sending people to Miami for training and then the harvesting will be done on site," overseen by Klein and Elizabeth Read, who runs the cell-processing facility. The intellectual partnership between Harlan, Kirk, and their Diabetes Research Institute collaborators will persist with regard to islet transplant studies as the protocols enter clinical trials at both centers.
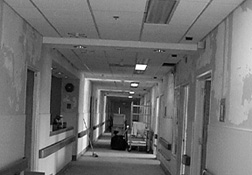 |
 |
 |
|
11
East Before the Change: The long view (left) and up close (center
and right)
|
||
Meanwhile, work has begun on 11 East to transform what had been a pediatrics unit until last December into a transplantation unit that will house eight to 10 beds and accompanying facilities. The first task, barely underway in January, was to make the bathrooms more accommodating to persons with handicaps. May 1 is the target opening date. "Everyone’s pulling to make it. The Division of Engineering Services is being very creative," says Henderson. He estimates that the first year of the project will cost the Clinical Center about $2 million—for renovation, reagents, personnel, patient care, and equipment, including an ultrasound machine and some cell-processing refinements. The outlay was endorsed by all the institute directors who will be contributing to the project’s upkeep through the "school tax" mechanism.
The program’s total cost was estimated at about $5 million a year in a "news brief" that went out on the NIDDK web site late last year. That "guesstimate," Spiegel says, encompasses posttransplant outpatient costs, which any institution seeking UNOS certification is expected to be prepared to pay and which includes a "huge amount" for immunosuppressive medications.
"In the best of all
possible worlds, however, there won’t be any," he observes, and the
costs will be much lower. ![]()