
| T H E N I H C A T A L Y S T | J A N U A R Y - F E B R U A R Y 1999 |
|
|
|
NEW CLINICAL RESEARCH PLANSLEAP SPACE AND SPECIALTY BARRIERS |
by Celia Hooper |
 |
|
John
Gallin
|
While architects and bulldozers are scratching out the physical groundwork for the new Clinical Research Center (CRC), others are at work on the programmatic groundwork, hatching plans they hope will fill the place with exciting, state-of-the-art clinical research.
With space as tight and precious as ever, proposed schemes for optimizing use of the new CRC combine creative leveraging of extant resources with selective, targeted additions of new money and positions. Three such initiatives, all at different stages of implementation, are the first waves of program changes for Clinical Center Director John Gallin. They include establishing "Centers of Excellence" in particular areas of clinical research, fostering collaborations between clinical and laboratory scientists, and establishing new partnerships with local hospitals and medical centers.
Background
In an address to a joint meeting of the scientific and clinical directors on November 4 last year, Gallin made it clear that it’s not just the lure of an attractive new building that’s spurring plans to bring top-flight clinical research to the Clinical Center. It’s also the old bugbears of declining patient enrollment, appropriate resource planning, and clinical researchers’ morale—issues sketched out in the Straus report in 1997 (see The NIH Catalyst, May–June 1997).
Marching orders for NIH must be to increase patient census while sustaining top-quality science and improving planning, Gallin said. This is no easy task because the patient population at any given time is based on the prior projections from each of more than a dozen institutes and divisions—their best estimates of numbers of protocols and patients to be recruited. These estimates are translated into demand for Clinical Center resources, forming the basisor hiring Clinical Center support staff and making quantity purchases of supplies. In the face of such uncertainties as marketplace forces and political winds, institutes have tended to overestimate clinical research needs—as they did, by 17 percent, last year—rather than underestimate and risk insufficient support of an approved protocol.
Reversing the downward trend in patient enrollment would be easier to accomplish if it weren’t for limitations on lab and office space—just hire more clinical investigators and get them rolling on some new research protocols. When the new CRC is completed in 2002, there will be some net additional space. Researchers from the old corridors of Building 10 will be relocated to the new building, allowing for some decompression and new recruitment. Space allocations in the new CRCwill rest on careful selection criteria, including the merits of the research, the need for proximity to patients, and the need for proximity to other clinical research programs in the CRC. Ultimately, creaky infrastructure will be decommissioned and closed.
Imaging Alliance
Of the proposed solutions for expanding the use of the Clinical Center, farthest along toward implementation is a project that emerged indirectly from discussions 18 months ago with administrators at Johns Hopkins University’s medical school in Baltimore. In Hopkins’ pursuit of a partnership with Suburban Hospital—which is just across Old Georgetown Road from NIH—the university leaders let both Suburban and NIH know that they would be much more interested in the relationship with Suburban if the hospital, in turn, had a close relationship with NIH. The Hopkins executives subsequently left the university for jobs elsewhere, but the idea of a partnership between Hopkins, Suburban, and NIH stuck. The most conspicuous areas for cooperation are with Suburban, says Gallin, particularly in hospital services NIH lacks, such as the emergency room.
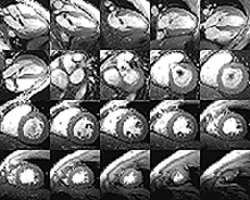 |
|
Montage of
a whole heart with a valve error, as captured by magnetic resonance imaging—an
instrumental piece of the collaboration between NHLBI and nearby Suburban
Hospital
|
Meanwhile, NHLBI investigators Bob Balaban and Andrew Arai had been developing multimodal cardiovascular imaging instruments and techniques and using them to image chronic heart disease patients in the Clinical Center. Recognizing an opportunity to apply the techniques to Suburban’s emergency room patients with acute myocardial infarction, Balaban became the project officer for what blossomed into a three-way partnership between Suburban’s emergency room, NHLBI, and NINDS.
To study patients with heart attacks, the heart institute has bought a powerful magnetic resonance imaging (MRI) instrument designed by Balaban’s lab and General Electric for one-stop, multimodality imaging of the beating heart with excellent 1-mm resolution. The instrument will be installed at Suburban and, without having to move patients to a series of imaging instruments, doctors there will be able to conduct one 45-minute scan to view the anatomy of the heart, its perfusion—how much blood is getting to each part—how well each section of the myocardium is beating, and the viability, scarring, and salvageability of all parts of the heart. Balaban says the imaging will allow doctors "to do a much better job in making decisions in triaging patients with acute chest pain"—for example, whether to catheterize a patient, send him or her home, or give thrombolytic therapy.
 |
|
Measurement
of cardiac contraction using a grid-tagging method
|
While Suburban’s patients will receive state-of-the-art imaging, Balaban’s lab will have research access, for the first time, to patients with acute heart disease. The new instrument, which should be up and running by the end of April, will give Suburban instant cachet as one of fewer than a dozen medical facilities in the country with the latest in heart-imaging technology.
Balaban is elated about the partnership and says his colleagues at Suburban feel the same way. "They are very excited. This is their first major collaboration with NIH. So far it looks like a win-win situation for both sides of Old Georgetown Road." Balaban adds that he thinks the program would serve well as a model for partnerships with other hospitals and academic centers in the area. "Through these arrangements, NIH could expand its influence on medicine in the area," Balaban observes. "In the past, our research hasn’t had nearly the impact it should on the quality of medical care here. This is a step forward."
NINDS is taking a slightly different approach in its collaboration with Suburban Hospital. Rather than buying an entire MRI instrument based on Balaban’s fast-scanning techniques, they will buy a 20 percent share of a head-imaging instrument based on the innovative technology. NINDS will then launch a protocol at Suburban to study stroke treatment, starting with an expected 200 stroke patients per year—again, one of the first intramural protocols to focus on emergency room patients. Balaban anticipates that some NIH time on the both the heart- and head-imaging instruments will be made available to institutes other than NHLBI and NINDS and scheduling of such use of the machines will be handled by the In-Vivo NMR Center steering committee.
Bench-to-Bedside Partnerships
Just emerging from the concept stage is a proposal (among the recommendations in the Straus committee report) to pair basic and clinical or translational scientists on research projects that draw from each partner’s strength. In December, Deputy Director for Intramural Research Michael Gottesman issued a call for statements of interest in such a program, which would provide up to three years of support for bench-to-bedside collaborations. Investigators could come from any institute or pair of institutes, and must include the active involvement of both a lab-based and a clinic-based investigator, with sign-offs on the work coming from at least one scientific director and one clinical director. "Much to our delight," Gallin reports, "we had a tremendous response" within a few weeks of the call, and 44 submissions were reviewed. Gallin and Gottesman winnowed these to 10 and will request formal proposals for support of up to $100,000 per year from an NIH-wide pot—possibly money saved by cost-cutting measures at the Clinical Center.
Stephen Straus, chief of the Laboratory of Clinical Investigation in NIAID, has been conducting clinical research in collaboration with scientists throughout NIH for 20 years and points out that the practice of pairing clinical and basic researchers from different institutes has been going on for years. But he has high hopes that the new program and infusion of money "will bring together lab and clinical investigators in a new way. This program makes a positive statement that NIH will encourage this kind of work," Straus says. Both he and Gallin expect that the institutes will offer support to good bench-to-bedside proposals that are not selected for central funding.
Centers of Excellence
Still in planning stages, the proposal for "Centers of Excellence" in clinical research is conceptually the most dramatic change for NIH. The inspiration for such centers comes from regional medical facilities, which are increasingly attempting to corner markets in certain medical specialties. This approach broadens the geographic area from which the hospitals draw patients and income. Gallin observes that the managed care industry questions why they would refer patients to NIH "rather than Mass General, [Johns] Hopkins, or a local institution. We have to have something special to offer and can with our centers."
NIH’s centers, as described by NIDDK’s Jake Liang and Straus in a recent proposal, would draw on traditional areas of NIH research strength—autoimmune diseases, behavioral medicine, or hepatitis, for example—and perhaps build upon the models established by current interinstitute training programs in genetics and endocrinology. Gallin envisions providing some central space and staff for the centers and recruiting or designating current NIH staff who are leaders in the fields to run the programs, which would stand at the center of a swarm of basic, translational, and clinical research, training, and shared lectures and rounds. Efforts are underway to identify the best potential research areas to cultivate as Centers of Excellence.
Gallin views the Centers of Excellence as the most exciting of the innovations at hand. "Once we have these centers, I could see some serious use of them to advertise nationally what we do at the Clinical Center," he says. Increased visibility would likely result in the spillover of new referrals and congressional interest to all of the intramural clinical research programs, he predicts.
Other Advances
Beyond these three proposals, Gallin sees other programmatic improvements in the works. The year 1999 will witness the launch of a major new emphasis on pain and palliative care at the Clinical Center, for example. After a campus-wide summit conference in November, NINDS, NIDCR, and the Clinical Center are working in concert both to improve management of pain for patients and to support pain research by the institutes. The main recommendation to emanate from the summit, Gallin says, was that a "comprehensive service in pain and palliative care" be organized. He expects to recruit physicians and nurse practitioners for the service this spring and to launch it by this summer. Ultimately, the team will include expertise in psychiatry, neurology, pharmacology, oncology, social work, and ethics, as well as pain assessment and management and the handling of chemotherapy complications.
One small experiment underway is in the Clinical Center’s hiring of a part-time consultant in internal medicine. In the past, the individual institutes have largely been responsible for identifying outside specialty consultants to assist with ancillary medical problems arising for protocol patients. Institutes frequently draw on one another’s expertise to handle these problems, as when NCI, for example, turns to NIMH practitioners for help with psychiatric issues arising in the treatment of a cancer patient. Gallin says that the Medical Executive Committee requested that he identify an internist who could provide consultation to several institutes that did not have access to that expertise. His selection was Fred Gill, a seasoned Bethesda physician who was on staff at NIAID before going into private practice. Gallin says he’ll review the results of the experiment in six months and see how well Gill’s consultations have satisfied institute "customers."
The most expensive change underway at the Clinical Center at the moment involves infrastructure rather than programs, Gallin says. That would be the new Clinical Research Information System, or CRIS. Costing about $30 million, CRIS will provide a comprehensive, integrated administrative and research information system—with all the bells and whistles. In addition to supporting a new and desperately needed activity-based cost-accounting system, which will help improve planning and finances, the system will permit the storage and delivery of all types of images, including anatomic, radiologic, and those derived from tests and functional and structural imaging of everything from the retina to the colon. CRIS will seamlessly link scheduling, protocol mapping, and connections between labs responsible for patient tests and specimen analysis. The project is expected to take three years, Gallin says.
So Far, So Good
Will the plans and changes
spark the spiritual renaissance so fervently sought by clinical leaders at NIH?
Straus says he, like others, is taking a wait-and-see stance, but he does detect
change in the air. "The sense I have is that there is a cautious optimism
at this point. The dialogue from NIH is very encouraging now, but clinical investigators
are waiting to see changes actually taking place," Straus says. The most
positive signs he sees already are in recruitment. "For the first time
in a long time, there are a large number of recruitments for investigators to
do real patient-oriented research. There was little or none of that before.
I am encouraged." The best news, Straus says from his view on some of the
search committees, is that "We are getting good applicants. This has always
been a phenomenal place to come to do research. Now they are coming not just
for the research resources but for the new building–it’s the physical
evidence of the new NIH commitment to patient-oriented research and that is
appealing to new applicants." ![]()