 |
 |
 |
||
David Tweedie and summer intern Ryan Short |
Tweedie with Nigel Greig |
The new Biomedical Research Center, Baltimore |
| TH E N I H C A T A L Y S T | JU L Y – A U G U S T 2008 |
| | |
In today's highly specialized scientific community, usually one lab makes a therapeutic chemical, another lab analyzes whether it will bind to a specific target, still another lab tests the compound on cells for real effects. The NIA Drug Design and Development Section does it all; and if the lab's recent research on TNF-α inhibitors continues to prove fruitful, researchers here are hoping for a breakthrough for the treatment of Alzheimer's, Parkinson's and other debilitating diseases of the central nervous system.
TNF-α, or tumor necrosis factor alpha, is implicated in a multitude of conditions, including rheumatoid arthritis and cancer, as well as many neurodegenerative diseases. And TNF-α lowering agents are being prescribed as therapeutic agents with somewhat mixed success.
What's new from NIA is the attempt to design drugs that can cross the blood-brain barrier to test the potential value of anti-TNF-α therapies for neurological diseases. Cell biologist and pharmacologist David Tweedie and medicinal chemist Weiming Luo lead this work under the guidance of Section Chief Nigel Greig.
 |
 |
 |
||
David Tweedie and summer intern Ryan Short |
Tweedie with Nigel Greig |
The new Biomedical Research Center, Baltimore |
Double-edged sword
TNF-α is a cytokine released by immune cells that induces inflammation. TNF-α is needed to provide a protective action to fight infections and to induce repair mechanisms to heal damaged tissues. TNF-α is released by microglial cells, which are brain-resident macrophage-like cells and the main line of immune defense in the central nervous system.
But TNF-α can be a double-edged sword. Although needed for proper immune responses, too much TNF-α for too long can exacerbate conditions such as Alzheimer's and Parkinson's diseases, Tweedie said. Once TNF-α is released in the brain, it can activate surrounding microglial cells, which causes them to unleash more TNF-α, creating a perpetuating cycle.
Whether too much TNF-α is the cause or the effect of various diseases remains to be determined. What is known, Tweedie said, is that "too much TNF-α makes the environment in the brain much more hostile for the neurons."
State of TNF-α therapy
Other researchers have already demonstrated that reducing the biological effects of TNF-α has beneficial effects in the clinical setting of Alzheimer's disease. TNF-α can be captured by biological therapeutic approaches before it promotes inflammation; one such example is the biological agent Enbrel® (Etanercept). Alzheimer's patients treated with the drug demonstrate measurable improvements in cognitive abilities within a matter of hours. These treatments are a strong indicator that TNF-α is a real effecter in the progression of Alzheimer's disease, Greig said.
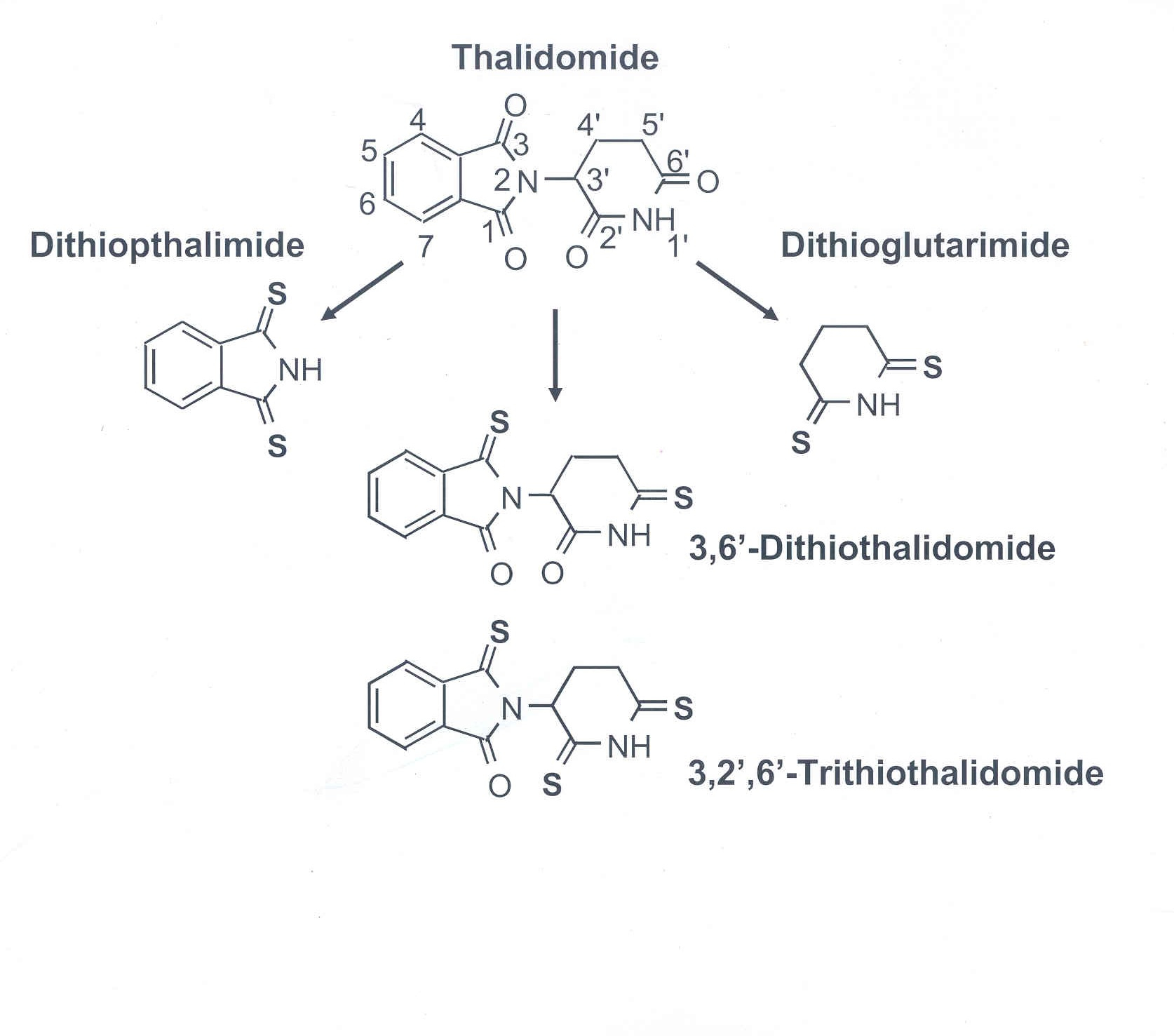
But there's a downside: This type of drug therapy cannot cross the blood-brain barrier and must be injected into the spine at regular intervals. This kind of invasive treatment can be painful for the patient, not to mention costly. (One treatment involves injecting patients and then placing them upside-down.) Tweedie's approach uses analogues of the small molecule thalidomide to inhibit TNF-α. Specifically, he adds sulfur groups to the rings of thalidomide to generate thiothalidomides in order to assess potential increases in the drug's effectiveness. Unlike current biological treatments in clinical trials, a major advantage of thalidomide is its ability to cross the blood-brain barrier. Thalidomide and its analogues appear to work by destabilizing the messenger-RNA levels of TNF-α instead of binding to the secreted protein. "Rather than capturing TNF-α before it hits its target, we are essentially turning off the faucet at the source," said Greig.
Nevertheless, some TNF-α is needed for normal function in the body. "Just enough is a good thing," said Tweedie. "Also if we switch off the synthesis of TNF-α too soon, it may cause more harm than good to the organism. We need to understand the therapeutic window for a given situation to be able to minimize those potential dilemmas."
The purpose of the inhibitors is to down-regulate the over production of TNF-α, not prevent its action in the body entirely. Tweedie's group has shown that their inhibitors do not completely prevent all TNF-α from being synthesized and secreted. This fact could mean less potential side effects from the drugs if used in a clinical setting.
 |
 |
 |
||
Weiming Luo |
Tweaking thalidomide |
Thalidomide and friends |
Thalidomides' infamy... and legacy
Thiothalidomides seem like the perfect candidates for therapeutics, but their parent compound has a tarnished reputation. Over 10,000 children were born with severe birth defects during the late 1950s and early 1960s from thalidomide prescribed to their mothers to relieve morning sickness during pregnancy. The drug was banned shortly afterwards.
Researchers from Israel discovered in the early 1960s that thalidomide reduced inflammation, but it was not until the 1990s that TNF-α was discovered as the target. Later the drug was approved as a treatment for certain types of cancer and an inflammatory condition caused by leprosy, yet tight regulations remain on who can get access to these meds.
These regulations seem less worrisome to Tweedie's cause, for the NIA is looking to use thiothalidomides to treat people with diseases associated with aging, at a time when women are past child-bearing age.
Greig's lab has found so far that the analogues with most promise for use as therapeutics are 3'6'-dithiothalidomide, which has two sulfur groups, and 3,2',6'-trithiothalidomide, which has three added sulfur groups to the thalidomide backbone. These compounds work to lower TNF-α levels 20 to 30 times better than thalidomide, without any toxicity.
Thalidomide shows no anti-TNF-α activity at the low concentrations that are effective for the analogues. Direct measures of the analogues effectiveness show reductions in the TNF-α RNA and secreted protein levels in a range from 28 to 92 percent, depending of the concentration of drug used. Strong supportive results like these indicate great potential for these candidates.
Drug discovery, one-stop shopping
The creation of new drug compounds requires many levels of interaction between the scientists. Luo and Tweedie discuss which analogues they will make based on the success of the previous compounds. Luo synthesizes the compounds in the chemistry lab and verifies their structure with techniques such as chromatography, mass spectrometry and nuclear magnetic resonance. Once the verification is complete, he passes the drugs onto Tweedie.
Tweedie initially tests his thiothalidomides for toxicity in vitro using mouse macrophage-like cells. He uses various assays to judge for cellular stress and viability based on the presence of cytoplasmic proteins detected in the culture media in which the cells are grown.
Once he finds inhibitors that cause minimal cell stress, he tests the effectiveness in vivo. Tweedie gives the TNF-α inhibitors to rodents, then he exposes the animals to LPS, which is a component found in bacteria cell walls that cause a large activation of the immune system. He checks for reduced levels of TNF-α RNA and secreted protein in the circulating blood plasma and in the central nervous system of rats treated with the inhibitors, for these markers indicate the effectiveness of the inhibitor.
Through a whole series of experiments, Tweedie determines the most effective concentration of each thiothalidomide for inhibition of TNF-α. By combining the toxicity data with the biological activity data, Tweedie "is the filtering system and [the one] who decides which drugs will go on to the next step," Greig said. Candidate inhibitors are then sent to NIA collaborators who study various aspects of Alzheimer's disease in either in vivo or in vitro models systems. They test the drugs for positive effects in the animals on a cellular level and on a cognitive level, acting essentially as "rodent psychologists."
Based on the success of the disease models, Tweedie and his collaborators will choose one of the drugs that act on a specific central nervous system disease to submit a package for patent rights. Although the thiothalidomides are disease non-specific in their action, Greig hopes they will target Alzheimer's or Parkinson's disease, for there is a portion of the population in real need of treatments.
Greig describes his lab as "an integrated assembly line because of the daily interactions and constant feedback." This type of interaction is what makes the lab so successful, he said, and is also responsible for the quick turn-around time between designing a chemical to determining whether it will be potentially useful as a therapeutic.
In addition to the sulfur-modified thalidomide, other TNF-α inhibitor analogues are in the works. But perhaps only Tweedie decides if they make the cut.
Further reading:
"TNF-α Inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets," Tweedie et al., Current Alzhheimer Research, 4: 378-385 (2007).
"Thiothalidomides: Novel isosteric analogues of thalidomide with enhanced TNF-α inhibitory activity," Zhu et al., Journal of Medicinal Chemistry, 46:5222-5229 (2003).