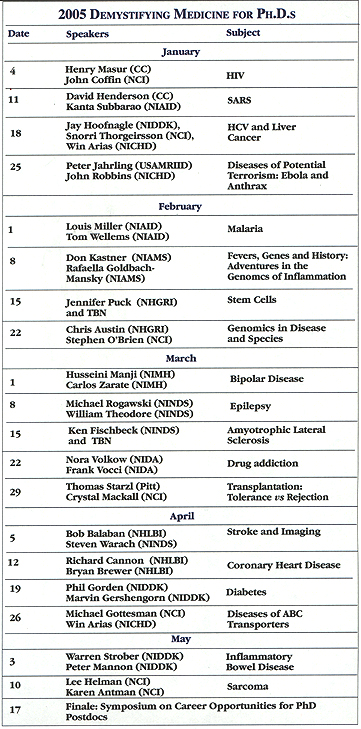
The popular Demystifying Medicine course will be offered again in 2005. The course aims to bridge the gap between Ph.D.s trained in basic science and the medical problems to which their skills and insights could be applied. Presentations of patients and pathology are accompanied by state-of-the-art analyses of related basic and clinical science.
Starting January 4 and ending May 17, the course will be held every Tuesday from 4:00 to 6:00 p.m. in the Building 50 ground-floor auditorium (Rooms 1227 & 1233). All presentations will be videocast.
The course is geared to graduate and medical students, clinical and Ph.D. fellows, and staff. Background information and handouts will be available.
Those seeking academic credit for the course can register with FAES; otherwise, registration is at the Listserv.
Participants who attend at least 75 percent of the sessions and complete a web-based final examination will receive a certificate. The course schedule follows and can also be found at the website.